Effect of Bone Marrow Cell Collection Techniques and Donor Site Locations on In-Vitro Growth of Bone Forming Cells
Article information
Abstract
Purpose
This study evaluated the influence of bone marrow cell collection techniques and donor site locations on the in-vitro growth of bone-forming cells.
Methods
Sixty six samples of bone marrow cell collections (BMCC) or bone marrow aspirates (BMA) from 15 patients were obtained. Thirty eight samples for culture were composed of 23 BMA from 7 tibial condyles and 16 ilia, with the other 15 BMCC from the contralateral ilia. The other 28 samples were used for the analysis of alkaline phosphatase activities. After counting total cell number, mesenchymal stem cells (MSC) obtained from samples were incubated for 14 days. Alkaline phosphatase staining was used to count the number of stained colonies to show osteogenic differentiation.
Results
The average MSC counts of BMA from tibial condyles and ilia were 1.42×106 and 7.35×106 respectively, with 4.80×106 from ilial BMCC (p=0.010). MSC cultures could not be produced from tibial condyles in all 7 samples. However, 9 of 15 BMCC samples and 9 of 16 ilial BMA samples were successfully cultured (p=0.018). The average of cell counts in the successful cultures was 7.92×106, whereas that in the failed cultures was 2.85×106 (p=0.000). Multiple regression analysis showed that colony count was associated with the patient's age and total cell numbers, but not with collection methods such as BMCC or BMA (p=0.000, R=0.648, beta; age=-0.405, cell number=0.356). The discriminating formula indicated that more than 5.25×106 cells were needed for successful culture.
Conclusions
For successful cultures in vitro and for grafts, the total number of collected bone forming cells is more important than donor sites or collection methods. For young patients, grafting of bone-marrow-derived osteoprogenitor cells is promising.
Introduction
New minimally invasive techniques for collecting bone marrow derived cells from various donor sites1-4, such as metaphysis of long bones, allow for in vitro and in vivo bone formation. Aspiration volume from the bone marrow5, harvesting technique6-8, donor site location,9 and the age-related decline10-14 can affect the in vitro growth of osteoprogenitor cells. Although bone marrow aspirate is the most common, bone marrow cell collection from the ground bone sludge and reaming debris of the long bone can also provide vital mesenchymal stem cells15. Reaming debris may also be an alternative to bone tissue grafting.
Here, we clarified the influence of bone marrow collection techniques and donor site locations on the growth of osteoprogenitor cells in vitro.
Materials and Methods
Fifteen patients who underwent spinal fusion using autogenous iliac bone grafts were recruited for the study. Ten patients were male and 5 were female. The mean age (±SE) at the time of surgery was 43±20 years old (range, 13 to 70 years old). Bone marrow cell collections (BMCC) were obtained from bone marrow sludge during harvesting of bone grafts in group I. Bone marrow aspirates (BMA) were obtained percutaneously from the iliac crest on the contralateral side in group II and from the proximal metaphysis of the tibia in group III. Sixty six samples of BMCC or BMA were obtained. Thirty eight samples for culture were composed of 23 BMA from 7 tibial condyles and 16 ilia, with the other 15 BMCC from the contralateral ilia. The other 28 samples were used for the analysis of alkaline phosphatase activity. Five ml of each sample was obtained using a 5 cc syringe containing heparin solution and a bone marrow aspiration needle. Two ml of the heparinized bone marrow suspension was used for the study on colony formation containing alkaline phosphatase staining and 2 ml for the alkaline phosphatase activity assay. For culture of bone marrow derived cells16, 2 ml of each bone-marrow suspension was mixed with two volumes of saline and one volume of Ficoll and centrifuged at 1,500 rpm for 10 minutes. The buffy coat was isolated and washed with two volumes of saline. After calculating the total number of cells using a hemocytometer, each sample was plated in a 100-mm-diameter dish. Cells were incubated in 8 ml Dulbecco's Modified Eagle Medium (DMEM) (GIBCO, Carlsbad, CA, USA) containing 10% fetal bovine serum (GIBCO), 10 nM dexamethasone, and 1% antibiotic/antimycotic (GIBCO) at 37℃, 95% O2 and 5% CO2. Culture media were changed on day 7 and then every 3 days for 1 week. On day 14, the total number of colonies stained with alkaline phosphatase was counted and alkaline phosphatase activity was measured in each culture dish. For alkaline phosphatase staining, the medium was removed and the cell layer was rinsed with phosphate buffered saline (PBS) two times. Cells were incubated with 2% paraformaldehyde for 30 minutes and then rinsed with PBS three times at 25℃. Cells were then incubated with 1.5 ml naphthol AS-BI alkaline solution (Sigma, St. Louis, MO, USA) with fast red violet LB for 15 minutes, and the total number of red alkaline phosphatase-positive colonies in each 100-mm dish was manually counted. For the alkaline phosphatase activity assay, the medium was removed and the culture dish was rinsed with PBS three times. The cell layer was disrupted with 1 ml of 0.5% Triton X in 25 mM glycine-NaOH and then 100 µL of solution was transferred to a microcentrifuge tube for the assay. Fifty microliter p-nitrophenyl phosphate (Sigma) was added to microcentrifuge tube, which was incubated for 30 minutes at 37℃ in 95% O2 and 5% CO2. The reaction was stopped with 50 µL of 2N NaOH and absorbance was read immediately after incubation at 405 nm in a plate reader.
The chi-square test, t-test and one-way ANOVA were used to confirm group differences. Multiple regression and discriminant analysis were used to extract factors associated with successful culture.
Results
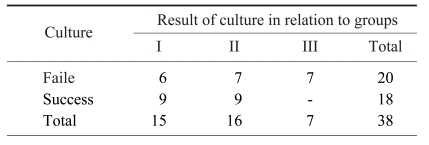
Average cell counts in group I, II and III were 4.80×106, 7.35×106, and 1.42×106, respectively (p=0.010) (Table 1), with a significant difference only between groups II and III. MSC counts were variable within each group. BMCC cell counts from ilia in group I ranged from 150,000 to 13,120,000. BMA cell counts from ilia in group II ranged from 19,500 to 15,000,000, and those from tibial condyles in group III ranged from 13,000 to 5,920,000.

Average numbers of cell count associated with the method of collection and donor site of bone marrow cells
Multiple regression analysis suggested that the age, donor site location, and collection method would contribute to cell count values (Table 2). Cell counts were increased in younger patients, allowing for sufficient harvesting from the proximal metaphysis of the tibia. Percutaneous aspiration of bone marrow was more efficient than bone marrow cell collection from ground bone or bone sludge.
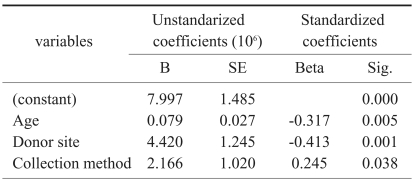
Selected variables according to the result of stepwise method of multiple regression-dependent variable=cell count
Six of 15 BMCC samples and 7 of 16 BMA samples from the ilium failed in culture, whereas all 7 samples of MSC aspirated from the tibial condyle failed in culture (p=0.018) (Table 3).
Average cell counts of successful and failed cultures were 7.92×106 and 2.85×106, respectively (p=0.000). Average colony counts in group I and II in cases of successful culture were 210 and 184, respectively (p=0.761) (Table 4).

Average numbers of alkaline-phosphatase (ALP)-positive colony count associated with the method of collection and donor site of bone marrow cells
Multiple regression analysis indicated that colony count was associated with the patient's age and total cell numbers, but not with collection methods (p=0.000, r=0.648, beta; age=-0.405, cell number=0.356) (Table 5). The discriminating formula indicated that 5.25×106 or more cells were needed for successful culture (Table 6).
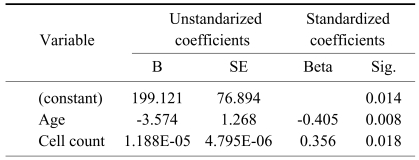
Selected variables according to the result of stepwise method of multiple regression-dependent variable=numbers of count of colony
Average alkaline phosphatase activity in the cultured mesenchymal stem cells in group I, II, and III were 0.593, 0.862, and 0.087, respectively, with group III significantly lower than the other two groups. Multiple regression showed that alkaline phosphatase activity was related to cell count, but not age, donor site location, or collection method (Tables 7 and 8).
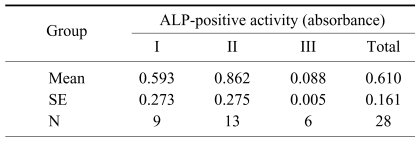
Average numbers of alkaline phosphatase (ALP) activity associated with the method of collection and donor site of bone marrow cells
Discussion
To avoid donor site morbidity, minimal invasive percutaneous surgical techniques, bone substitutes, and induction materials are used. Bone marrow aspiration is the most common5, where bone marrow and marrow cells are collected using a bone drill or biopsy trephine7 to supply bone forming cells to the nonunion site of the long bone and the bone substitute. Reaming debris of the long bone is an alternative substitute for bone grafts, but the outcomes from this process are not clearly understood. The metaphysis of long bones, such as the tibia1 and the radius and coronoid processes of the ulna can be local alternative sources near the lesion. Thus, donor site location9, harvesting technique6-8 and age-related decline of osteoprogenitor cells11-14, as well as aspiration volume, can affect in vitro and in vivo growth of bone forming cells. The relationships between these factors are not well understood.
Here we show that total colony formation in vitro was related to the patient's age and the total number of MSC obtained, with age more important in the regression analysis. In contrast, donor site location was more important than age or collection method for total cell count. Thus, the metaphysis of long bones is not a good source of stem cells, especially in elderly patients. The mean cell count (±SD) of all samples of patients younger than 20 years old was 7.497×106 (range, 2.260×106 to 13.600×106) and all samples except one were cultured successfully, meaning that younger patients have more abundant and more active marrow cells. Thus, bone marrow derived osteoprogenitor cell grafts derived from young patients have greater potential for survival, with more cells needed in elderly donors.
The metaphysis of long bones are an alternative source of marrow cells. However, we found that all tibia samples failed in culture. Most also yielded low cell counts (less than 1.5×106) and one sample with a total cell number of 5.920×106 still failed in culture. Thus, the ilium is considered to be a better source for bone marrow derived stem cell.
Reamed bone debris and bone sludge seemed to be advantageous for in vivo growth because of induction materials contained in the bone matrices, but they did not provide sufficient stem cells for in vitro growth.
Conclusions
For successful in vitro culture and grafting, the total number of collected bone forming cells is more important than donor site or collection method. In young patients, the graft of bone-marrow-derived osteoprogenitor cells is promising. The ilium is the best source of osteoprogenitor cells, but future work may improve yields from long bones.