Model of Induced Leakage of Polymethylmethacrilate Inside Epidural Space and Prevertebral Muscles During Vertebroplasty in Pigs: Clinical, Macroscopical, and Histological Study
Article information
Abstract
Study Design
Experimental study in animals.
Purpose
Study the clinical behavior of animals after an induced leakage of cement during vertebroplasty in pigs. Study the distribution of polymethylmetacrilate inside the epidural space and prevertebral muscle. Study the histological findings of the spinal cord and muscles, which contact with cement.
Overview of Literature
Although vertebroplasty has a low rate of complication, leakage of cement is highly frequent. There is paucity, in how cement is distributed inside the spinal canal and what occurs when soft tissue comes into contact with polymethylmetacrilate.
Methods
We performed vertebroplasty on six pigs. We performed a leakage of cement into the epidural space and into prevertebral muscles. Two weeks later we performed an anatomic evaluation regarding the spreading of polymethylmetacrilate and a histological analysis of soft tissues that came into contact with it.
Results
No clinical alterations were observed. We observed a laminar distribution of the cement surrounding dura mater, and creating a fusiform cavity inside muscles. Spinal cord was normal in all the animals. In dura mater, we observed: synovialmetaplasia, inflammatory reaction, crystal deposits, and giant-cell-reaction. In muscles, we observed: inflammatory reaction, crystal deposits, giant-cell-reaction, muscular atrophy, fibrosis, and synovial metaplasia.
Conclusions
The spinal cord was normal; it is likely that dura mater and cerebrospinal fluid are responsible to isolate neural structures from cement. Dura mater and muscle showed similar histological changes than other publications. Synovial metaplasia was observed in dura mater and muscles that came into contact with cement. The pulsatile rubbing between the tissue and cement could be responsible of this phenomenon.
Introduction
Vertebroplasty is a useful technique for treating osteoporotic compression fractures, vertebral malignancy, and symptomatic vertebral hemangiomas. Excellent rates of pain relief and improved quality of life have been cited throughout various studies [1-7].
Although vertebroplasty have a low rate of complications, 1% to 3% for osteoporotic fractures, 2.5% for hemangiomas, and up to 10% for metastatic vertebral lesions [8-12], leakage of cement out of the bone towards the epidural space, vessels, or the perivertebral tissues is highly frequent. It has been observed in up to 70% of the cases, fortunately without severe clinical complications [11-16].
Existing literature poorly describes the behavior of the cement when it leaks from the vertebrae and also poorly describes the histological changes of the soft tissue like dura mater and muscle when they come into contact with polymethylmethacrilate. Most of the studies, in humans and in some animals, have focused on bony histological changes, describing inflammatory reaction, foreign body reaction and bone necrosis [17-23].
The high frequency of leakage of polymethylmethacrilate during vertebroplasty and the paucity of information especially on the macroscopic distribution of the cement in the epidural space and the potential histological alterations of the spinal soft tissues, like the spinal cord, the dura mater and the perivertebral muscles, that may come into contact with the cement when it leaks. Considering this, we decided to set up a reproducible model of epidural and prevertebral leakage of polymethylmethacrilate for vertebroplasty in pigs, to study the cement-spreading pattern into the spinal canal and muscles, the potential histological changes in the spinal soft tissues and try to relate it, with the clinical behavior of the animals after the procedure.
Materials and Methods
For this study we obtained approval from the local institutional review board for animal experiments.
1. Animals
We used six female, three-month-old Large White Landrace pigs weighing 30 to 35 kg, obtained from a professional stockbreeder.
We followed the rules for assessment and alleviation of postoperative pain and end point or euthanasia criteria for pain behavior, paraplegia, and dying animal models [24,25].
The surgical procedure began with a preanesthetic sedation (ketamine 10 mg/kg, intramuscular one time) administered in the stall, followed by general anesthesia that was administered by orotracheal intubation as follows: anesthetic induction: azaperone 2 mg/kg intramuscular one time, atropine 1 mg intramuscular one time, etomidate 3 mg/kg intravenous, then, muscle relaxation with pancuronium 0.2 mg/kg intravenously, followed by oro-tracheal intubation.
The maintenance of the anesthesia was done with isofluorane 0.8% to 1% plus oxygen 40% and pancuronium 0.2 mg/kg every 30 minutes intravenously plus fentanyle 0.005 mg/kg intravenously every 30 minutes.
Like prophylactic antibiotics we used one dose of 1 g intravenous ampicillin.
For the surgical procedure the animals were placed on a radiolucent surgical table in a prone position.
The animal's back was shaved, disinfected with clorhexidine, and draped with surgical fields, exposing the dorsal mid line.
We used C-arm radioscopic guidance (Powermobil Siemens, Erlagen, Germany). For vertebroplasty we used a conventional 15 cm, 13-gage vertebroplasty trocars (Osteo-Site, Cook, IN, USA), vertebroplasty cement (Vertebroplastic, De Puy Spine, Raynham, MA, USA) and 2 mL. Luer-locked syringes for the cement injection. The cement was mixed and injected following the manufacturer's instructions.
To select the vertebral level we choose the vertebra most easily viewed under antero-posterior C-arm imaging, currently between L1 and L3.
To place the cement in contact with prevertebral and myeloradicular tissues, we performed two types of cement escape: one was an epidural leak, for this, we performed a percutaneous right pedicle cannulation of a central lumbar vertebra, later on, under the antero-posterior radiological view, the tip of the trocar penetrates the pedicle from the lateral to the medial cortex following a 30°-angle trajectory, a lateral view was used to corroborate the epidural placement of the tip (Fig. 1).
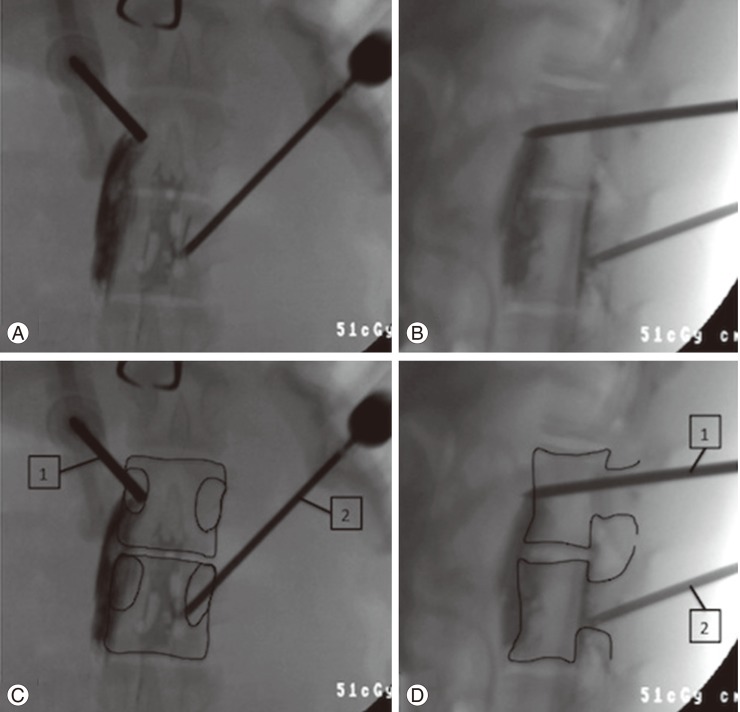
(A, B) Intraoperative X-ray. (C, D) Diagrams enhancing the vertebral cortex. (A, C) Frontal view, upper vertebra, trocar number 1 (prevertebral leak) the trocar enters the pedicle in a near right angle trajectory. Inferior vertebrae, trocar number 2 (epidural leak): the cannula enters the pedicle in a 30-degree inward angle, penetrating the medial cortex of the pedicle. (B, D) Lateral view, superior vertebra, cannula number 1 (prevertebral leak): we can observe the tip of the trocar penetrating the anterior cortex of the vertebral body. Inferior vertebrae, cannula number 2 (epidural leak): the tip of the cannula stays behind the posterior cortex of the vertebral body. (A, B) We can observe the polymethylmethacrilate leaking antero laterally to the upper vertebra in a fusiform way (prevertebral leak). In the inferior vertebrae we can observe the leakage in the spinal canal in a laminar way (epidural leak).
To induce a prevertebral leak, we performed a percutaneous access on the left pedicle of an adjacent vertebra. Under the antero-posterior radiological view the trocar penetrates the center of the pedicle in a near 90°-angle trajectory, later on, using a lateral radiological view, we penetrated the anterior cortex of the vertebra (Fig. 1).
Under antero-posterior and lateral radiological views, one milliliter of vertebroplasty cement was injected in both vertebrae.
Finally the skin was disinfected with clorhexidine and sutured with surgical silk 3/0.
After surgery the animals were placed in a warm cage and received postoperative analgesia (buprenorphine 0,005 mg/kg subcutaneous, one time). The animals were placed in their cages with free food and water delivery, and were observed daily for limb motor function using the Tarlov scales [26], sphincters function, and any pain behavior or end point criteria [24,25].
2. Euthanasia
The animals were sacrificed two weeks after the surgery with an intravenous injection of pentobarbital sodium 18%, 200 mg/kg.
Later on we performed sample acquisition and histological analysis: the lumbar spine of each pig was harvested from a posterior approach, maintaining the anterior muscles.
The pedicle insertion points were identified. An extensive and careful laminectomy was performed to expose the cement and the dural sac (epidural leak), after the laminectomy we took a set of photographs to document the distribution of the polymethylmethacrylate in the epidural space. Later on we collected the dural sacs by cutting the roots near the foramina; the cement inside the psoas muscle (prevertebral leak) was found by manual palpation and then, was exposed. We took photographs of every specimen.
All the areas of the dural sac and of the muscle that had been in contact with the cement were marked with Chinese ink to make it easy to find those areas during microscopal observation. We use Chinese ink because it is widely used to mark tissues for histological analysis without inducing any change on dead tissues (Fig. 2).

(A) During removal of the spinal cord, we can see cement near the axila of the root (arrow). (B) The area of the cord in contact with the cement was marked with ink (arrow).
All the samples were fixed in formaldehyde.
Every tissue marked with Chinese ink were then dehydrated with ethanol, fixed in wax, cut in 5 µm slices with a microtome, and stained with H&E.
Under light microscopy, a senior pathologist inspected the samples, searching for the presence of inflammatory reaction, tissue necrosis, foreign body reaction, or any other histological alteration.
Results
1. Clinical results
Pig number 5 presented a transient tachycardia during the surgery without any clinical consequence.
Pig number 6 died during the procedure after the cement injection. Necropsy did not reveal the cause of death. We especially looked for venous or pulmonary cement embolisms.
None of the pigs presented any neurological impairment after the procedure.
No superficial infections or healing problems were observed in the whole group of animals.
2. Macroscopic result
1) Epidural leak
The five vertebrae with epidural leak showed a laminar distribution of the polymerized cement that surrounded the dural sac and the root ipsilateral to the injection side, as a thin layer of cement (Fig. 3A). In one case the cement practically surrounded the cord a full 360° (Fig. 3B). Wide areas of the dural sacs that were in contact with the cement were successfully identified and were marked with Chinese ink. Those areas did not show any evidence of macroscopic changes.
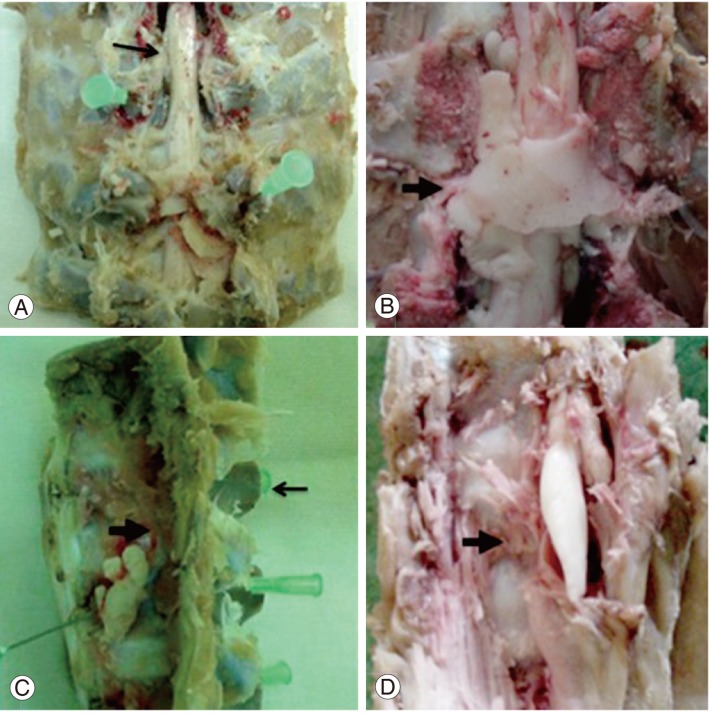
Macroscopic findings. (A) Antero-posterior view of the spine. Pig number 1: post laminectomy, the needles show the trocars entering the pedicles, the arrow shows the cord. (B) Antero-posterior view of the spine. Pig number 3: post laminectomy, the arrow shows the spinal cord completely surrounded by cement. (C) Lateral view of the spine. Pig number 2: the thick arrow shows the cement in a prevertebral location; the thin arrow shows the spinous process. (D) Lateral view of the spine. Pig number 5: cement is observed in the prevertebral area inside the psoas (arrow).
2) Anterior prevertebral leak
The cement inside the muscles was distributed in a fusiform way by creating cavities. The inner layer of those cavities had a pseudo membrane aspect (Fig. 3C, D).
3. Histological results
1) Epidural leak
On dura mater we observed synovial metaplasia in three of five pigs, an inflammatory reaction (presence of lymphocytes, neutrophyls, and eosinophyls) in four of five, crystal deposits in four of five pigs and a giant cell reaction around crystal deposits in four of five pigs (Fig. 4).
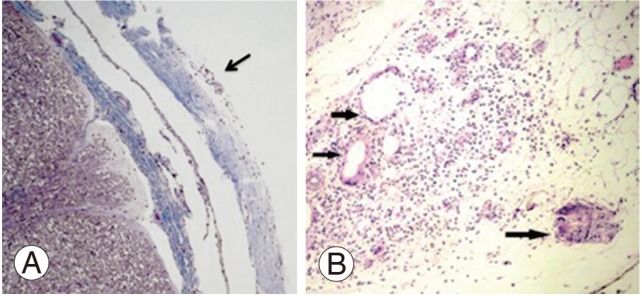
Spinal cord and dura mater histology. (A) Normal spinal cord. The arrow indicates the dura mater with an inflammatory reaction (H&E, ×2). (B) A close-up of the dura mater reveals a lot of lymphocytes; arrows indicate giant cell formations (H&E, ×4).
The histological study of the spinal cord of the five animals, were completely normal (Fig. 4).
2) Anterior prevertebral leak (muscular findings)
In four out of the five pigs, inflammatory reaction was observed.
All the samples demonstrated crystal deposits, giant cell reaction, muscular atrophy, and synovial metaplasia (Fig. 5).
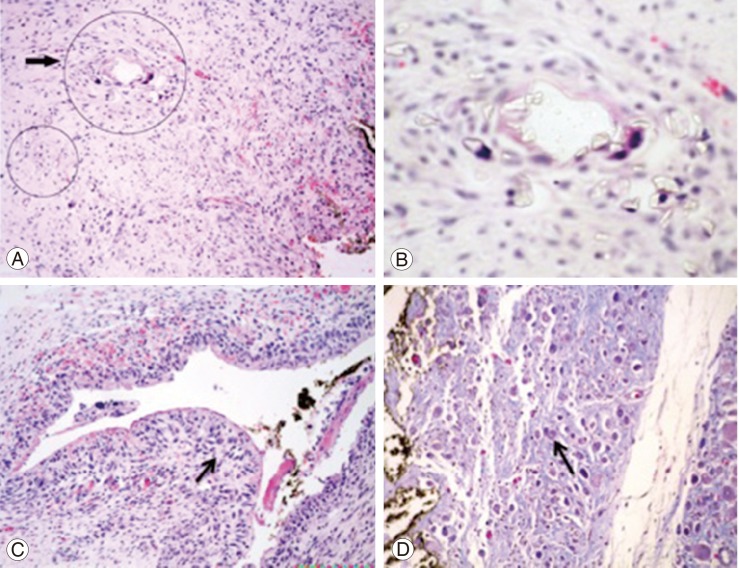
Histology of the prevertebral muscle. (A) Muscle with inflammation, the arrow and circles indicate giant cell formation (H&E, ×4). (B) Close-up, we can observe a giant cell and many crystals inside and around it (H&E, ×40). (C) The arrow shows synovial metaplasia of the muscle near the ink mark (H&E, ×4). (D) Muscle fibrosis and atrophy (arrow) (H&E, ×20).
Discussion
We consider that the vertebroplasty model in pigs is very similar to the procedure that we normally used in our patients: the morphology of the vertebra and the size of the animal enabled us to perform the procedure using the same trocars that we used in humans. Moreover, the surgical setting is the same as the one we use for our patients.
Despite massive leakage of cement into the epidural space (Figs. 2, 3), none of the animals presented neurological impairment after two weeks of observation. These findings are comparable to clinical series, in which radiological leaks are very common but neurological impairment that requires surgery are highly infrequent [11]. A possible explanation of this phenomenon is that the liquid cement is distributed in a laminar way around the dura mater, while is modeled by the pulsatile movements of the dura mater.
On the dura mater and the prevertebral muscles we observed an inflammatory reaction (presence of lymphocytes, neutrophyls, and eosinophyls), a foreign body reaction, and fibrosis. Those changes are probably related to the toxicity of polymethylmethacrilate, the presence of Barium crystals and the exothermal reaction of the cement during its polymerization [17-23].
We did not find necrosis on the dura mater, a probable explanation is that the polymerization temperatures may not reach the threshold of tissue necrosis in a live animal, probably because the epidural vessels and the cerebro spinal fluid are able to dissipate the heat and prevent the exothermal damage [23,27].
We also did not observe any changes in the spinal cord, probably because the dura mater and cerebrospinal fluid are sufficiently able to isolate the neural structures from the inflammatory reaction and from the exothermic reaction of the curing cement.
On the muscular tissue we observed slight atrophy, fibrosis, and no necrosis. These changes differ from the changes described on bone, where the necrosis is more frequent. This could be because liquid cement may require less pressure to distribute itself, inside soft tissue than inside the bone; in fact, an in-vitro study in cadaveric vertebras showed an increase of 13.6 folds on average over the base line of bone pressure during the injection of polymethylmethacrilate [28].
Synovial metaplasia was observed in both dura mater and muscles that had been in contact with the cement; the pulsatile rubbing between tissue and the cement may be responsible for this histological adaptation. As far as we know, synovial metaplasia is not previously described, probably because all the literature available focuses on changes in the bone.
Conclusions
The vertebroplasty model in pigs is very similar to the procedure in humans. This study shows that the way the cement distributes itself inside the epidural space and in perivetrebral muscles does not induce a significant clinical alteration. The laminar way in which the cement is distributed in the epidural space may be what prevents a mechanical lesion of the spinal cord. We did not observe any changes in the spinal cord, probably because the dura mater and cerebrospinal fluid are sufficiently able to isolate the neural structure from the polymethylmethacrilate. We observed an inflammatory reaction, a foreign body reaction, fibrosis, and synovial metaplasia on the dura mater and on the prevertebral muscles.
Acknowledgments
For this study we obtained financial assistance from the PIUNA fund of the University of Navarra, Spain.
Notes
No potential conflict of interest relevant to this article was reported.