Comparative Radiographic Outcomes of Lateral and Posterior Lumbar Interbody Fusion in the Treatment of Degenerative Lumbar Kyphosis
Article information
Abstract
Study Design
Retrospective case–control study.
Purpose
To compare surgical invasiveness and radiological outcomes between posterior lumbar interbody fusion (PLIF) and lateral lumbar interbody fusion (LLIF) for degenerative lumbar kyphosis.
Overview of Literature
LLIF is a minimally invasive interbody fusion technique; however, few reports compared the clinical outcomes of conventional PLIF and LLIF for degenerative lumbar kyphosis.
Methods
Radiographic data for patients who have undergone lumbar interbody fusion (≥3 levels) using PLIF or LLIF for degenerative lumbar kyphosis (lumbar lordosis [LL] <20°) were retrospectively examined. The following radiographic parameters were retrospectively evaluated preoperatively and 2 years postoperatively: segmental lordotic angle, LL, pelvic tilt (PT), pelvic incidence (PI), C7 sagittal vertical axis, and T1 pelvic angle.
Results
Nineteen consecutive cases with PLIF and 27 cases with LLIF were included. There were no significant differences in patients’ backgrounds or preoperative radiographic parameters between the PLIF and the LLIF groups. The mean fusion level was 5.5±2.5 levels and 5.8±2.5 levels in the PLIF and LLIF groups, respectively (p=0.69). Although there was no significant difference in surgical times (p=0.58), the estimated blood loss was significantly greater in the PLIF group (p<0.001). Two years postoperatively, comparing the PLIF and LLIF groups, the segmental lordotic angle achieved (7.4°±7.6° and 10.6°±9.4°, respectively; p=0.03), LL (27.8°±13.9° and 39.2°±12.7°, respectively; p=0.006), PI–LL (19.8°±14.8° and 3.1°±17.5°, respectively; p=0.002), and PT (22.6°±7.1° and 14.2°±13.9°, respectively; p=0.02) were significantly better in the LLIF group.
Conclusions
LLIF provided significantly better sagittal alignment restoration in the context of degenerative lumbar kyphosis, with less blood loss.
Introduction
Adult spinal deformity cases have increased along with the population’s advancing age, so there is a greater need for less invasive surgical treatments that can provide sufficient spinal alignment restoration for aged patients [1]. In particular, sagittal alignment imbalance is strongly associated with poor quality of life; therefore, it is important to create a well-balanced sagittal alignment via surgery [2]. Among the various surgical treatments available, lumbar interbody fusion is effective. The procedure provides neural decompression, stabilizes painful mobile segments, restores lordosis, and corrects deformities. As a result, this surgical technique is frequently used to treat degenerative lumbar kyphosis [3]. Various lumbar interbody fusion techniques have been described, including posterior lumbar interbody fusion (PLIF) [4], transforaminal lumbar interbody fusion (TLIF) [5], and anterior lumbar interbody fusion (ALIF) [6]. Recently, lateral lumbar interbody fusion (LLIF) has gained popularity [7]. LLIF is a lateral transpsoas approach to a disk, which allows less invasive surgical exposure, while avoiding major vessels and permitting the placement of larger interbody cages than PLIF or TLIF [7,8]. By allowing a greater disk height and spinal alignment restoration via using larger cages, LLIF could become a desirable alternative to other interbody fusion techniques.
However, surgical decision-making for treating degenerative lumbar kyphosis is complicated by few comparative effectiveness studies that directly evaluate various approaches. Reportedly, LLIF can improve local sagittal alignment [9,10]; however, its impact on global or spinopelvic alignment is still unclear [11]. In addition, no reports have compared the radiographic outcomes for PLIF and LLIF in the context of degenerative lumbar kyphosis. Thus, the superior surgical method for achieving sagittal alignment correction is still unknown. Therefore, the current study’s objective was to compare the radiographic outcomes and surgical invasiveness of PLIF and LLIF in the context of degenerative lumbar kyphosis.
Materials and Methods
1. Study design
We retrospectively reviewed the radiographic records of consecutive patients with lumbar kyphosis (lumbar lordosis [LL] <20) who underwent PLIF between 2009 and 2013 and LLIF between 2013 and 2016, with the approval of the Konan Kosei Hospital institutional ethics committees (IRB approved no., 29-030 [0315]). This study was a retrospective study, and then informed consent of patients were not obtained when the current study started. The patients to whom any of the following applied were excluded from the study: (1) age <20 years; (2) an involvement level of less than 3; (3) upper instrumented vertebrae <T8; (4) osteotomy grade >2 [12]; (5) less than one year of follow-up; and (6) other disease entities present, such as tumors, traumas, or infections. Ultimately, 46 patients were included: 19 consecutive cases with PLIF (average age, 70.2±5.9 years; 9 men, 10 women) and 27 consecutive cases with LLIF (average age, 73.2±6.7 years; 11 men, 16 women). Neurological symptoms were evaluated using the Japanese Orthopedic Association criteria (JOA score) for low back pain (full marks of 29 points).
2. Radiographic analysis
Preoperative and one-year postoperative anteroposterior and lateral radiographs of the lumbar spine and whole-spine were obtained in an upright, standing position. The radiological parameters included (1) segmental lordotic angle: Cobb’s angle between the upper and the lower end plates of each fused segment; (2) LL: Cobb’s angle between the upper end plates of both L1 and S1; (3) pelvic tilt (PT): the angle between the line linking the midpoint of the upper end plate of S1 and the center of the hip joint and vertical line; (4) pelvic incidence (PI): the angle between the line linking the midpoint of the upper end plate of S1 and the center of the hip joint and the line vertical to the upper end plate of the sacrum; (5) C7 sagittal vertical axis (SVA): the distance between the posterosuperior corner of S1 and the vertical line from the C7 body center; and (6) T1 pelvic angle (TPA) [13]: the angle between the line from the femoral head axis to the centroid of T1 and the line from the femoral head axis to the middle of the S1 superior end plate.
3. Surgical procedure
The general technique for PLIF has been previously described [14]. After a complete posterior neural decompression, we performed interbody fusion using two carbon fiber cages (DePuy AcroMed Corp., Raynham, MA, USA) at 80.3% of the levels and one boomerang cage (Medtronic Corp., Memphis, TN, USA) at 19.7% of the levels. These cages were filled with local bone obtained during decompression.
As for the surgical technique for LLIF, extremely lateral lumbar fusion using CoRoent cages (NuVasive Inc., San Diego, CA, USA) was performed [7,15]. We performed bilateral pedicle screw fixation approximately 1 week after LLIF.
4. Statistical analysis
IBM SPSS Statistics ver. 21.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. The mean values are presented as mean±standard deviation. Student t-test and Fisher’s exact test were used to compare the two groups. A p-value of <0.05 was considered statistically significant.
Results
There were no significant differences in the patients’ preoperative backgrounds between the two groups in terms of age (p=0.12), sex (p=0.77) (Table 1) and preoperative neurological severity, as assessed by the JOA score (p=0.57) (Fig. 1). The preoperative radiographic parameters were not different either: LL was 10.7°±5.3° (PLIF) and 9.1±6.9° (LLIF) (p=0.40), SVA was 90.3±41.5 mm (PLIF) and 95.0±49.2 mm (LLIF) (p=0.74), PI–LL was 36.5°±9.6° (PLIF) and 37.7°±10.6° (LLIF) (p=0.70), PT was 29.5°±8.7° (PLIF) and 29.9°± 9.4° (LLIF) (p=0.88), and TPA was 32.8°±14.1° (PLIF) and 30.7°±11.4° (LLIF) (p=0.58) (Table 1).
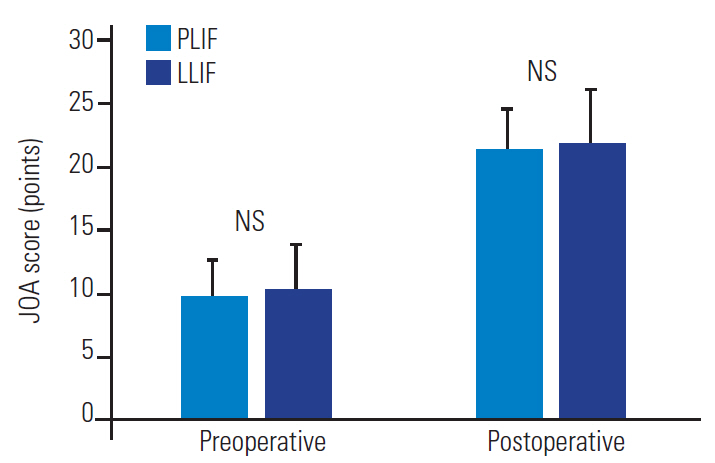
Pre- and postoperative JOA scores. There were no statistically significant differences at either pre- (p=0.57) or 2 years post-surgery (p=0.61). PLIF, posterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion; JOA score, Japanese Orthopedic Association score; NS, not statistically significant.
The mean fusion level was 5.5±2.5 and 5.8±2.5 levels in the PLIF and LLIF groups, respectively (p=0.69). Although there was no significant difference in surgical times (517.7±232.9 minutes (PLIF) and 481.0±161.6 minutes (LLIF) (p=0.58), the estimated blood loss was significantly greater in the PLIF group than in the LLIF group (2,676.2±1,652.0 mL and 991.1±652.9 mL, respectively; p<0.001). Preoperative JOA scores were 9.8±3.1 (PLIF) and 10.4±3.4 (LLIF) (p=0.57), and postoperative JOA scores were 21.4±3.4 (PLIF) and 22.0±4.3 (LLIF) (p=0.61) (Fig. 1).
The mean interbody cage height was 9.8±1.4 mm (PLIF) and 9.6±1.6 mm (LLIF) (p=0.39) and the cage lordotic angle was 1.3°±2.2° (PLIF) and 10.0°±0.0° (LLIF) (p<0.001). For lumbar kyphosis correction, a grade 2 osteotomy was used in 10 cases (52.6%) in the PLIF group and in three cases (11.1%) in the LLIF group; thus, osteotomy was used significantly more often in the PLIF group (p=0.003).
In the LLIF group, the radiographic parameters were assessed after LLIF and before posterior fixation. LL was 28.4°±9.5° (p<0.001), PI–LL was 11.0°±15.6° (p<0.001), and PT was 14.5°±14.4° (p<0.001), all of which were significantly better than the corresponding preoperative levels.
In terms of postoperative radiographic parameters after posterior fixation, the achieved segmental lordotic angles were 7.4°±7.6° in the PLIF group, but these angles were significantly greater in the LLIF group: 10.6°±9.4° (p=0.03). In addition, LL, PI–LL, and PT were significantly better in the LLIF group than in the PLIF group. LL was 27.8°±13.9° (PLIF) and 39.2°±12.7° (LLIF) (p=0.006), PI–LL was 19.8°±14.8° (PLIF) and 3.1°±17.5° (LLIF) (p=0.002), and PT was 22.6°±7.1° (PLIF) and 14.2°±13.9° (LLIF) (p=0.02) (Table 1). Regarding the other radiographic parameters, there were no statistically significant differences: SVA was 57.5±44.2 mm and 35.5±49.8 mm (p=0.13) and TPA was 31.8°±8.8° and 23.6°±9.6° (p=0.06) in the PLIF and LLIF groups, respectively.
In the LLIF group, after LLIF but before posterior fixation, LL and PT had already been corrected to 62% and 66%, respectively, of their final total extent of correction.
1. Representative case of posterior lumbar interbody fusion
A representative PLIF case is a 78-year-old female patient with lower back pain and intermittent claudication, who presented with the following (Fig. 2A, B): a preoperative whole-spine standing radiograph showing an LL of 5°, PT of 19°, PI of 42°, SVA of 5.6 cm, TPA of 19°, and a coronal Cobb angle of 26° (Fig. 2C, D); then, a whole-spine standing radiograph at one year following PLIF with grade 2 osteotomy at L3–L4, L4–L5, and L5–S, with the radiograph showing an LL of 29°, PT of 16°, SVA of 9.5 cm, TPA of 18°, and a coronal Cobb angle of 2°.
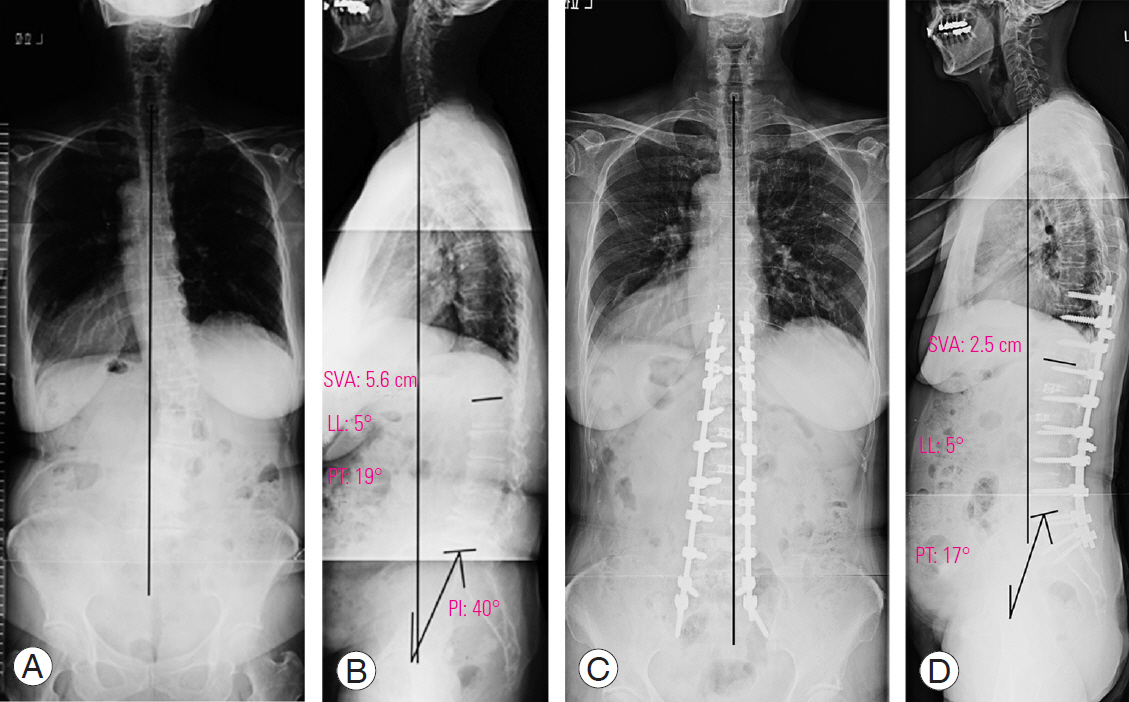
A preoperative, whole-spine standing radiograph in the anteroposterior (A) and lateral (B) view. A whole-spine standing radiograph at 1 year following posterior lumbar interbody fusion with grade 2 osteotomy at L3–L4, L4–L5, and L5–LS in the anteroposterior (C) and lateral (D) view. SVA, C7 sagittal vertical axis; LL, lumbar lordosis; PT, pelvic tilt; PI, pelvic incidence.
2. Representative case of lateral lumbar interbody fusion
A representative LLIF case is a 77-year-old female patient with lower back pain and right leg pain, who presented with the following (Fig. 3A, B): a preoperative whole-spine standing radiograph showing an LL of 8°, PT of 39°, PI of 52°, SVA of 11.1 cm, TPA of 44°, and a coronal Cobb angle of 37° (Fig. 3C, D); then, a whole-spine sitting radiograph after LLIF, with the radiograph showing an LL of 29°, PT of 18°, and a coronal Cobb angle of 14° (Fig. 3E, F); and a whole-spine standing radiograph at 1 year following LLIF and posterior fixation without grade 2 osteotomy, with the radiograph showing an LL of 55°, PT of 18°, SVA of 2.4 cm, TPA of 14°, and a coronal Cobb angle of 3°.

A preoperative whole-spine standing radiograph in the anteroposterior (A) and lateral (B) view. A whole-spine sitting radiograph after LLIF in the anteroposterior (C) and lateral (D) view. A whole-spine standing radiograph at one year following LLIF and posterior fixation without grade 2 osteotomy in the anteroposterior (E) and lateral (F) view. LLIF, lateral lumbar interbody fusion; SVA, C7 sagittal vertical axis; LL, lumbar lordosis; PT, pelvic tilt; PI, pelvic incidence.
Discussion
This is the first study comparing sagittal realignment after PLIF and LLIF in cases with degenerative lumbar kyphosis. Our results suggest that LLIF can provide a greater local lordotic angle and LL, and that the degree of PT correction can be significantly greater with LLIF. These greater sagittal realignments were achieved with fewer osteotomies and less blood loss. Thus, corrective surgery for degenerative lumbar kyphosis using LLIF appears to result in more effective interbody stabilization and correction with less invasiveness than PLIF.
To date, several lumbar interbody fusion approaches have been developed. ALIF permits placement of a large interbody cage which provides significant stability in an interbody space [3]. However, spinal surgeons are unfamiliar with this approach and there is a risk of complications, including major vascular or ureteral injury, ileus, and retrograde ejaculation in males. The PLIF/TLIF approach provides 360° fusion through a single posterior approach, which is familiar to spinal surgeons [4]. Therefore, this approach was the most frequently used in lumbar interbody fusion in the past decade. However, it carries a risk of iatrogenic paraspinal muscle damage, durotomy, nerve injury, and fibrous scarring around the dura. LLIF was developed by Pimenta in the late 1990s as a lateral endoscopic transpsoas retroperitoneal approach, and it was first introduced by Ozgur et al. [7] as a minimally invasive lumbar interbody fusion technique. The LLIF approach enables direct visualization of a wide area of the lateral aspect of the disk, permitting excellent disk space preparation and placement of a large interbody cage [7,8]. As a result, LLIF can effectively provide disk height restoration, which allows indirect neural decompression by enlarging the interbody space and increasing the neuroforaminal height [7,10]. In addition, posterior fixation, with a transpedicular screw supplementing LLIF, provides significant additional fixation stiffness and deformity correction. Therefore, this approach has been used to treat adult spinal deformities [16].
In adult patients with spinal deformities, spinal alignment changes, secondary to disk degeneration, are the main cause. Therefore, corrections at the interbody space are the most reasonable surgical approach [17]. Surgeons are better able to make corrections at the interbody space with LLIF than with PLIF/TLIF because inserting large cages is possible with LLIF [8]. In previous studies, LLIF resulted in improved local sagittal alignment [18]. In addition, an increase in disc height from 41.9% to 83% and an increase in foraminal height from 13.5% to 83% were observed after LLIF [19,20]. As for the segmental acquired lordotic angle achieved with LLIF, Anand et al. [20] reported an 8.1° increase in segmental lordosis in adult spinal deformity cases with LLIF, using a 10° cage. Ohtori et al. [21] investigated 80 patients (mean fusion level: 1.5 levels) and found that the acquired lordotic angle was 3.8° in LLIF with 6° lordotic cages. On the other hand, acquired segmental lordosis (0.1°–4.7°) following PLIF/TLIF was smaller than with LLIF [11,22], and it might be difficult to achieve sufficient segmental lordosis with the usual PLIF. Hence, in order to achieve greater lordosis, invasive spinal osteotomy or hyperwedge requires consideration when conventional PLIF is used [22,23].
There is currently no consensus on improvements in global sagittal alignment following LLIF. Acosta et al. [24] have published a retrospective report on a cohort of 36 patients treated with LLIF for degenerative lumbar diseases at 66 interbody levels with supplemental fixation of pedicle screws. In their study, significant regional segmental lordosis improvement was obtained; however, LL and global sagittal alignment were not significantly changed. On the other hand, in their multicenter study, Ha et al. [25] have retrospectively reviewed the radiographic parameters of adult spinal deformities and reported a 16° improvement in LL after LLIF, with an additional 5° increase after supplemental pedicle screw fixation. A correction effect was more easily detected in cases with preoperative hypo-LL or global sagittal imbalance. Therefore, statistical significance could be more easily obtained in those cases. Phillips et al. [26] reported surgical results for 107 adults with degenerative scoliosis treated by LLIF, with and without supplemental posterior fixation, and they noted that significantly greater LL had been achieved in cases with preoperative hypo-LL (9.9° LL increase) than in cases without preoperative hypo-LL (3.3° LL increase). Cho et al. [11] reported global sagittal balance improvement was achieved only in cases with preoperative sagittal imbalance. In this study, the radiographic outcomes with PLIF and LLIF were compared only for cases with hypo-LL, and the baseline radiographic data were more standardized than in previous studies. Under these conditions, LL and PT were found significantly greater in LLIF cases, indicating that LLIF (≥3-level fusion) can be an effective surgical treatment approach to sagittal deformity correction in lumbar kyphosis cases.
In the current study, an average increase of 10° in the local lordotic angle was obtained with LLIF, an increase on par with that obtained with PLIF supplemented by grade 2 osteotomy [12]. If a greater lordotic correction is needed with LLIF surgery, an anterior column realignment (ACR), or an additional osteotomy higher than grade 2 [12], should be considered. ACR involves severing the anterior longitudinal ligament and placing hyperlordotic cages; thus, a greater segmental correction can be achieved than with LLIF alone [27,28]. However, ACR carries the risk of damage to large blood vessels, suggesting that its appropriateness should be carefully considered. In cases with an additional osteotomy higher than grade 2 [12], surgical invasiveness becomes greater, as does blood loss; therefore, osteotomy levels should be carefully selected. Considering the current study results, the correction methods and need for ACR or posterior osteotomy to obtain an ideal LL need to be further investigated.
Some limitations in the current study should be acknowledged. First, this study was retrospective. Second, it was conducted using a relatively small number of cases. A large-scale prospective study is clearly needed. Greater sagittal realignment with less blood loss was achieved to a satisfactory degree in the current cases as well as in previous reports, suggesting that the results may not differ significantly in a larger-scale study. Finally, the time period when PLIF was performed was earlier, and our surgical target LL in PLIF surgery was slightly different from that in the LLIF group. Most PLIF surgeries were performed before 2012, when the Scoliosis Research Society-Schwab classification was published, so our surgical target LL was determined based on a 90° pelvic radius-S1 (PR-S1) angle [29]. The PR-S1 angle was reported by Jackson and Hales [29] in 2000. It was measured between the line from the posterior superior corner of S1 to the point on the middle axis of the femoral heads and a tangent line along the S1 endplate intersecting at the posterior superior corner of S1. Given PI was measured at the ‘midpoint’ of the S1 endplate, the 90° PR-S1 angle is close to PI. Therefore, our intraoperative LL goal was effectively PI in both the PLIF and LLIF cases. However, in reality, the 90° PR-S1 angle is slightly smaller than PI; therefore, the target LL in PLIF was approximately 3°–5° smaller than that in the LLIF group. However, there is another reason why an ideal LL was not obtained in the PLIF group. In the PLIF surgery, due to massive intraoperative blood loss, we could not successfully perform grade 2 osteotomy at many levels for geriatric patients older than 70 years. As a result, in the current study, there were a greater number of patients with PI–LL >10° in the PLIF group. This result could be a trade-off relationship between the volume of intraoperative blood loss due to osteotomy and the degree of postoperative LL, which is one of the difficult aspects of PLIF surgery.
Conclusions
In patients with degenerative lumbar kyphosis, LLIF provides better LL and PT than PLIF, with less blood loss and fewer additional osteotomies. Thus, LLIF is a useful surgical method for sagittal realignment with minimal surgical invasiveness.
Notes
Tokumi Kanemura is a consultant of Medtronic and Nuvasive. No potential conflict of interest relevant to this article was reported.
Author Contributions
Nakashima H: corresponding author, conception, design, and analysis; Nakashima H, Kanemura T, Satake K, Ishikawa Y, Ouchida J, Segi N, Yamaguchi H: acquisition of data; and Kanemura T, Satake K, and Imagama S: critically revising the article.