Are We Looking at a Paradigm Shift in the Management of Adolescent Idiopathic Scoliosis? Comprehensive Retrospective Analysis of 75 Patients of Nonfusion Anterior Scoliosis Correction with 2–5-Year Follow-up: A Single Center Experience
Article information
Abstract
Study Design
Retrospective cohort study.
Purpose
This study aimed to evaluate the clinical and radiological outcomes of nonfusion anterior scoliosis correction (NFASC) in patients with idiopathic scoliosis and comprehensively analyze its principles.
Overview of Literature
NFASC is a novel revolutionary motion-preserving surgery for idiopathic scoliosis. However, clinical data related to this procedure remain scarce, with no conclusive guidelines regarding case indications, proper technique, and possible complications.
Methods
This study included patients with adolescent idiopathic scoliosis (AIS) who were treated with NFASC for a structural major curve (Cobb angle, 40°–80°) with more than 50% flexibility on dynamic X-rays. The mean follow-up was 26±12.2 months (range, 12–60 months). Clinical and radiological data such as skeletal maturity, curve type, Cobb angle, surgery details, and Scoliosis Research Society-22 revised (SRS-22r) questionnaire were collected. Statistically significant trends were examined by post hoc analysis following repeated measures analysis of variance test.
Results
A total of 75 patients (70 females, five males) were included, with a mean age of 14.96±2.69 years. The mean Risser and Sanders scores were 4.22±0.7 and 7.15±0.74, respectively. The mean main thoracic Cobb angles at the first and second follow-up (17.2°±5.36° and 16.92°±5.06°, respectively) were significantly lower than the preoperative Cobb angles (52.11°±7.74°) (p<0.05). Similarly, the mean thoracolumbar/lumbar Cobb angle significantly improved from the preoperative period (51.45°±11.26°) to the first follow-up (13.48°±5.11°) and last follow-up (14.24°±4.85°) (p<0.05). The mean preoperative and postoperative SRS-22r scores were 78.0±3.2 and 92.5±3.1, respectively (p<0.05). None of the patients had any complications until the most recent follow-up.
Conclusions
NFASC offers promising curve correction and curve progression stabilization in patients with AIS, with a low risk for complications and preservation of spinal mobility and sagittal parameters. Thus, it proves to be a favorable alternative to fusion modality.
Introduction
Scoliosis is a spinal disorder that leads to a severely compromised quality of life and disability, affecting roughly 1 in 300 children [1]. In recent years, many innovations have been made for the surgical treatment of idiopathic scoliosis, especially with regard to a growing spine. Nonfusion anterior scoliosis correction (NFASC) is a new, revolutionary motion-preserving treatment method for adolescent idiopathic scoliosis (AIS) [2,3].
The NFASC, also known as anterior vertebral body tethering (AVBT), is based on the Hueter–Volkmann law [4]. In this procedure, compressive instrumentation on the convex side of a scoliotic curvature inhibits this side from growing while permitting the concave side to lengthen with growth; this mechanism progressively corrects the spinal deformity [5–7].
The gold standard for managing AIS remains to be spinal fusion, but it is associated with potentially negative consequences, such as a high risk for decreased spinal growth over the length of the fusion construct, decreased spinal mobility [8], adjacent disk degeneration [9], chronic back pain, and perioperative complications including neurological problems, wound infection, and excessive blood loss [10,11].
NFASC treatment for scoliosis has recently gained interest. It is similar to anterior fusion surgery wherein an internal brace construct is created, albeit without spinal fusion [12]. NFASC offers an option for correction without fusion; however, the technique is novel, and clinical data related to this procedure remain scarce. Thus, this study aimed to evaluate NFASC in patients with AIS at a tertiary care hospital in India, adding crucial scientific evidence in relation to the utility of this new surgery modality in these patients.
Materials and Methods
After obtaining institutional review board approval and informed consent from all patients, we collected data of patients with AIS who underwent NFASC. The inclusion criteria were as follows: structural major curve (40°–80°) and more than 50% flexibility on traction X-rays. The mean follow-up of the eligible patients was 26±12.2 months (range, 12–60 months).
Patient data were recorded until the latest follow-up. Demographic details along with the curve type by Lenke classification, and Risser and Sanders’ scores were analyzed. We also collected clinical and radiological data such as skeletal maturity, curve type, Cobb angle, and surgery details (blood loss and surgery duration). The radiological assessments included the preoperative and postoperative Cobb angles with the percentage of corrections for both main thoracic (MT) and thoracolumbar/lumbar (TL/L) curves, wherever applicable. Postoperatively, the follow-up data were collected at the time of the first erect radiograph, 6 months, 2 years, and at the most recent follow-up. We also compared the mean preoperative and postoperative Scoliosis Research Society questionnaire (SRS-22r) results [13]. Complications were also determined immediately postoperatively, as well as at all the follow-up time-points.
1. Surgical technique
Patients were placed in the lateral decubitus position, with the convex side up. With single lung ventilation, standard minithoracotomy was performed to permit improved three-dimensional evaluation of the anatomy and endorse safe bi-cortical screw placement. Those who underwent instrumentation at L4 needed a mini-open approach from the retroperitoneal end. Vertebral levels were confirmed with C-arm fluoroscopy in both the anteroposterior and lateral views. During the surgery, we positioned a three-prong staple on the vertebral body right in front of the rib head. Next, we tapped the screw hole and positioned the optimum-length screw. The remaining screws were placed identically. Generally, strategic screw positioning affects sagittal profile restoration posteriorly at the curve’s apex, where the vertebra is most rotated and anteriorly at the ends of the curve. This technique will derotate the apex by cord tensioning because the screws will readjust to the shortest distance between each other. By using staples, the screw will be better positioned in the anteroposterior aspect of the vertebral body [14].
Moreover, the Globus Philadelphia Reflect scoliosis correction system was used. In this system, the tether was passed in a distal-to-proximal manner and positioned into the screw heads. A pistol grip tensioning device was connected with the tether, allowing a graded compressive force up to 400 N2. The maximum intraoperative curve correction was attained by combining apical translation, derotation, segment compression, and sequential tether tensioning. During tether cord tensioning, we applied frontal pressure mainly at the apical region to achieve more coronal plane correction. Sequential tensioning was preferred instead of global tensioning method, as mentioned in several studies, with higher tensioning at the apical region to obtain maximum correction intraoperatively. The amount of tension needed depends on the extent of the curve, the potential for residual limited growth, and the analysis of curve flexibility on the traction X-ray, and this is guided intraoperatively under fluoroscopy to mainly horizontalize the lower instrumented vertebra.
Subsequently, we closed the incision and the chest meticulously without intercostal chest drain to promote early mobilization, reduced length of stay (LOS), and early pulmonary function recovery [15]. Postoperatively, the patients were strictly observed for possible pleural fluid accumulation. Aggressive chest physiotherapy was also provided. In our center, we do awake traction X-rays. Preoperative traction radiographs help in reasonably estimating postoperative correction for AIS patients undergoing NFASC [16]. It guides the surgeon intraoperatively for appropriate tensioning for adequate correction [17].
Perioperatively, we followed the Enhanced Recovery Pathway (ERP) (Fig. 1), which is a multimodal approach for improving the perioperative outcomes of patients by using specific evidence-based protocols for surgical care [18,19]. ERP includes epidural analgesic management with mobilization and initiation of triflowmetry from the day of surgery to approximately 4–6 hours postoperatively. Then, with gradual tapering of the epidural, rehabilitation is increased sequentially. Thus, ERP leads to early recovery and mobilization, reduced morbidity, decreased opioid consumption, shorter LOS, and overall improved patient satisfaction scores in patients with AIS undergoing NFASC.

Enhanced Recovery Pathway goals included improved pain control, reduced opioid-related complications, expedited early postoperative mobilization, and early transition to oral analgesics from patient-controlled analgesia. This results in an overall improvement in patient satisfaction scores in adolescent idiopathic scoliosis patients undergoing nonfusion anterior scoliosis correction.
2. Statistical analysis
Statistical data were analyzed using the IBM SPSS Statistical Software ver. 25.0 (IBM Corp., Armonk, NY, USA). The statistical difference in mean values across various time-points was assessed using repeated measures analysis of variance. The mean values between any 2 time-points were compared by post hoc analysis, wherever applicable. The preoperative and postoperative mean SRS scores were compared using paired t-test. A p-value of less than 0.05 was considered statistically significant.
Results
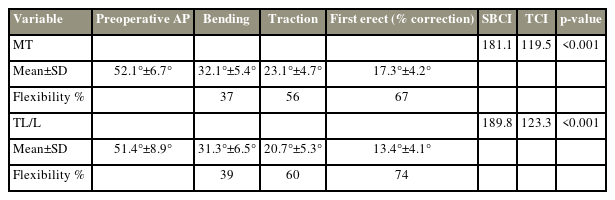
This study enrolled 75 patients (70 females, five males), with a mean age of 14.96±2.69 years. The mean Risser score was 4.22±0.7, whereas the mean Sanders’ score was 7.15±0.74. Thus, we found 10 immature and 65 skeletally mature patients. In the Lenke classification, majority (n=32, 42.22%) had type 5 curve, whereas 24 patients (35.50%) had type 1 curve. Table 1 summarizes the perioperative details.
Cranial and caudal instrumented levels were T5 and L4. Preoperatively, the mean MT traction angle was 23.07°±6.31°, whereas the mean TL/L traction angle was 20.72°±6.49°. The mean MT bending angle was 32.69°±6.81°, and the mean TL/L bending angle was 31.28°±8.91°. Further, the mean MT traction flexibility was 55.81%±0.09%, whereas the mean TL/L traction flexibility was 59.77%±0.09%. The right bending flexibility was 36.72%±0.12%, and the left bending flexibility was 38.88%±0.12% (Table 2).
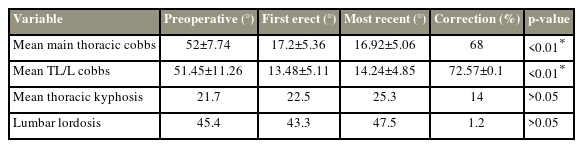
Postoperatively, as per our ERP, opioid epidural infusion was continued for 42.5±3.5 hours with morphine at 2 mg/24 hr and fentanyl at 2 mcg/hr with 0.12%–0.15% ropivacaine up to 48 hours. The mean MT Cobb angle at the first follow-up (17.2°±5.36°) and last follow-up (16.92°±5.06°) were significantly lower than the preoperative Cobb angle (52.11°±7.74°) by post hoc analysis (p<0.05). The mean TL/L Cobb angle at the first follow-up (13.48°±5.11°) and last follow-up (14.24°±4.85°) were also significantly lower than the preoperative TL/L Cobb angle (51.45°±11.26°) (p<0.05).
The mean correction in the MT and TL/L Cobb angles was 66.88%±0.09% and 73.6%±0.08% at the first follow-up, while it was 67.93%±0.08% and 72.57%±0.1% at the latest follow-up, respectively (Table 3). The mean values for thoracic kyphosis preoperatively, immediately postoperatively, and at 2 years were 27.20°±8.20°, 30.83°±5.85°, and 31.09°±6.06°, whereas those for lumbar lordosis were 48.92°±8.41°, 47.23°±7.58°, and 48.32°±6.06°, respectively (p>0.05). Pelvic incidence and pelvic tilt showed no significant changes from the preoperative period to 2 years of follow-up (p>0.05). Furthermore, the mean SRS-22r score was 78.0±3.2 preoperatively and 92.5±3.1 postoperatively (p<0.05). None of the patients had any complications until the latest follow-up.
Discussion
NFASC has led to a paradigm shift in the management of AIS, presenting a new option for orthopedic surgeons. The gold standard for scoliosis deformity correction is posterior scoliosis correction and fusion. However, the improved spinal alignment with fusion comes at the cost of permanent, iatrogenic regional ankylosis of the corrected segments, fewer motion segments, and increased risk of degenerative changes in the adjacent segments, causing secondary back pain. Hence, spinal motion preservation, particularly the lumbar spine, is a highly attractive goal.
Recently, NFASC has become a novel and popular technique in treating AIS. Its advantages include a minimally invasive incision, less blood loss, continuous spinal lengthening, and probably, final fusion avoidance, leading to a lesser chance for adjacent disk degeneration. Considering the use of a lateral intermuscular plane, posterior muscle endurance is retained, resulting in early recovery.
NFASC or AVBT has been applied for approximately 10 years as an off-label management modality, which has not allowed the extensive evaluation of the novel technique. However, in August 2019, the US Food and Drug Administration approved AVBT by exempting the humanitarian device, and skilled centers are now propagating their early-to-midterm outcomes via few research publications [13–15]. However, published data on NFASC outcomes in patients with AIS remain scarce.
This study evaluates the radiological and clinical outcomes with a technical note of NFASC in patients with AIS. Our participants aged 8–20 years, which appropriately represents the adolescent age group. Majority of the patients in the study had type 5 (TL/L) or type 1 (MT) curve. The study findings were focused mainly on the correction in the mean MT and TL/L Cobb angles at first erect, 6 months, 2 years, and then at the most recent follow-up. Both the mean MT and the mean TL/L Cobb angles were significantly reduced from the first erect radiograph to the most recent follow-up compared with the baseline (p<0.05), indicating that the curve significantly improved (Figs. 2, 3). However, the mean angles were comparable between the first follow-up and final follow-up (p>0.05), showing that the maximum significant improvement in the curve was at the first follow-up. The sagittal parameters of the curve were also analyzed, and NFASC was found to have a positive influence on thoracic kyphosis without having any detrimental effect on lumbar lordosis. As a result, most of the patients achieved normal physiologic kyphosis, and some maintained their preoperative alignment (Fig. 4). With the positive influence of NFASC on sagittal parameters of the spine, authors propose a hypothesis regarding the orientation of the intervertebral disk in the thoracic and lumbar spine. The intervertebral disk in thoracic spine converges anteriorly, whereas that in the lumbar spine is broader anteriorly. Baroncini et al. [20] support the positive influence of NFASC on the sagittal parameters of the spine; they hypothesized that in the thoracic spine, the coronally oriented facets favor the rotation of the motion segments rather than the flexion–extension, creating a posterior tension band effect and resulting in anterior shortening, and in the lumbar spine, the facet joints are oriented sagittally, thereby favoring flexion–extension over torsion, causing a relaxed posterior column leading to an anterior tension band effect [20].
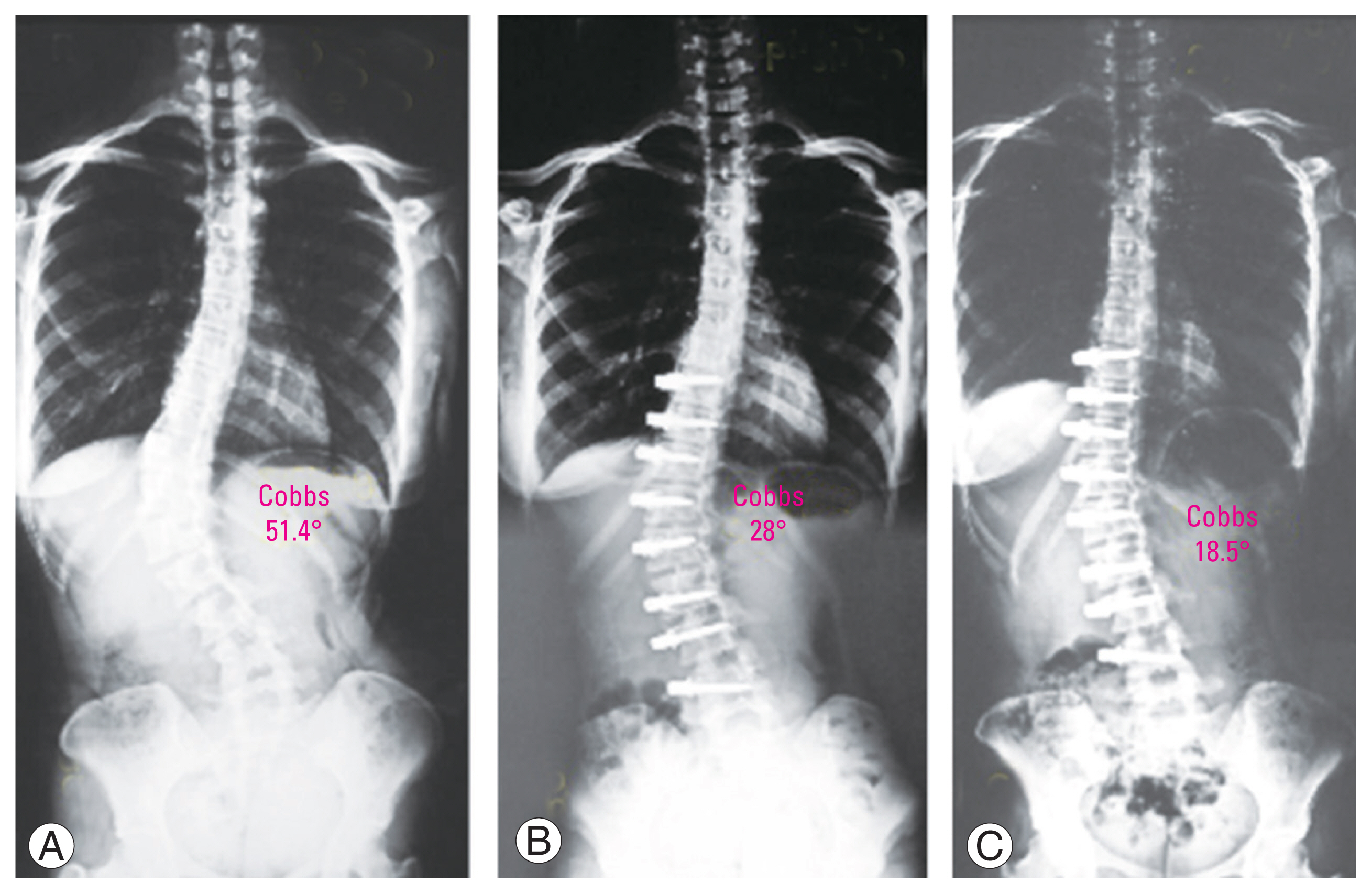
Case 1: 14-year-old girl with Risser 4 and Sanders 7 presented with Lenke 5CN. (A) Preoperative radiograph shows a Cobb angle of 51.4°. (B) Postoperative erect radiograph with Cobb measuring 28.0° (46% correction). (C) At 2-year follow-up, the deformity corrected to Cobb measuring 18.5°

Case 2: serial radiographs of 14-year-old girl with Sanders 7 and Risser 4. (A) Preoperative major thoracolumbar Cobbs (D10–L3) of 43.1°, (B) left bending film 20.4°, (C) traction of 11.6°, (D) first erect of 12.1°, (E) 1-year follow-up of 11.3°, and (F) 2-year follow-up of 6.1°
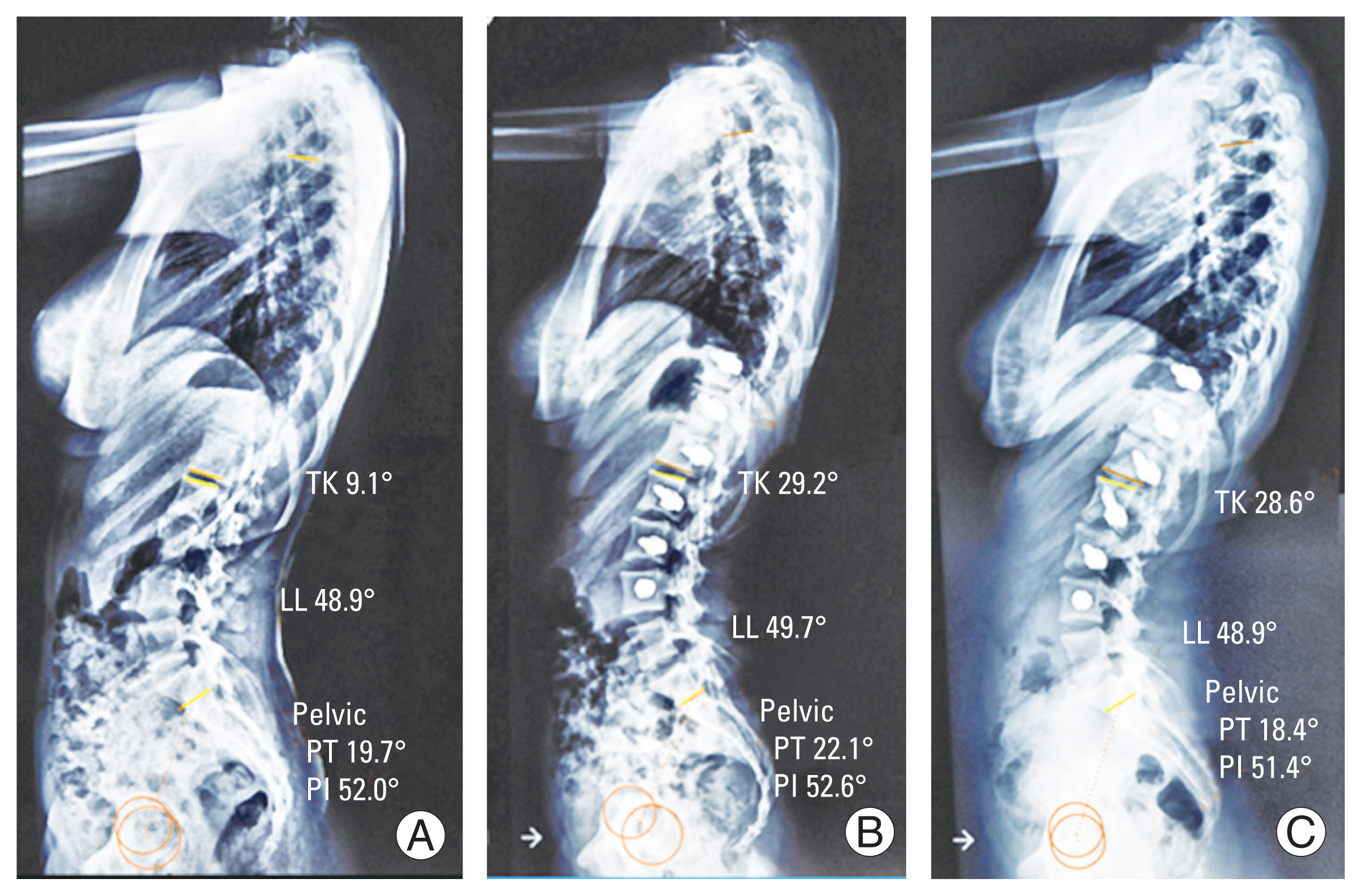
Case 3: serial lateral radiographs of case 3: 14-year-old adolescent idiopathic scoliosis patient showing positive influence on thoracic kyphosis (TK) along with maintenance of lumbar lordosis (LL). (A) Preoperative, (B) 6 months postoperative, and (C) 2 years postoperative. PT, pelvic tilt.
Surgery for AIS should consider the long-term effects. The main point is the quality of life of the patients within the following 50 or 60 years; in adult patients, sagittal balance has a strong correlation with health-related quality of life [21].
Given that all our patients were either Lenke curve type 1, 3, 5, or 6 with no structural proximal thoracic curve, none of them had any significant coronal imbalance or shoulder imbalance preoperatively. Even in patients with severe Lenke type 1, their shoulders were balanced postoperatively. Evaluation of shoulder balance has become essential in proximal thoracic curves, which were an exclusion criterion in our study.
The mean SRS-22r scores significantly increased (p<0.05), indicating that the patients had lesser pain and improved quality of life. In addition, none of our patients experienced any complication, indicating that the NFASC procedure is not only effective but also safe postoperatively.
In recent years, some studies had evaluated the NFASC outcomes, but the impact on deformity correction after AVBT has not been found to be uniformly similar in various studies [12,22–27] (Table 4). Hoernschemeyer et al. [22] evaluated the follow-up data between 2 and 5 years for 29 patients presenting with differing skeletal maturity at baseline and found a progressive correction in thoracic tethers, with a high success rate and low revision rates despite higher maturity at baseline. Newton et al. [23] assessed the AVBT outcomes at 2–5 years of follow-up in 23 patients who underwent NFASC in comparison with 26 patients who underwent posterior spinal fusion. Both the study groups displayed a progressive correction over the first 2 years. Though the patients who underwent NFASC had a lower deformity correction and a higher revision rate than those who underwent posterior spinal fusion, the postinterventional patient-reported outcomes were similar between these groups [23]. In the study of Cobetto et al. [28], progressive correction was noted in 20 patients after NFASC, from the preoperative mean 59° to immediate postoperative angulation of 37° and 23° at the follow-up conducted after 2 years. Further, Samdani et al. [12] investigated the 2-year outcomes of 11 patients who underwent NFASC and noted a higher correction at the initial follow-up, from the mean preoperative angulation of 44° to 20°, followed by a progressive correction to a mean angle of 14° at the follow-up after 2 years. However, Wong et al. [24] noted a mixed response in their analysis, after using a modality that avoided intraoperative tether tensioning; all the five enrolled patients obtained a Risser score of 0, but a progressive correction was noted in only two of the enrolled patients with open tri-radiate cartilages at surgery.
The authors of this study believe that through their experience, the best candidates for NFASC are patients with a major structural curve of 60°–65° with flexibility of more than 50% in awake traction X-rays.
Samdani et al. [12] showed reassuring safety results similar to the present study, but at 2 years, two of the 11 patients required revisions. Rushton et al. [29] followed up patients for more than 2 years; they noted complications in 22% of patients who underwent NFASC, and 15% needed revision. After NFASC, the disk health is maintained, whereas after posterior spinal fusion, adjacent disk degeneration occurs [30]. The present study had a minimum follow-up period of 2 years, and none of our patients had complications or revisions, probably because of the lower duration of follow-up. Hence, future studies with a longer follow-up can explore this aspect.
Our study has few limitations. The sample size was small, and the cases were evaluated at only one major orthopedic center and were not objectively measured in terms of the spinal range of motion. In addition, a mean 2-year follow-up is not adequate to evaluate the long-term outcomes; hence, a longer follow-up is essential to make any definitive statements about the accurate value of this novel technique.
Conclusions
NFASC offers a promising curve correction and stabilization of curve progression in patients with AIS, with a low risk of complications and preservation of spinal mobility and sagittal parameters. It is proven to be a favorable alternative to fusion modality. Future studies with a longer follow-up period are necessary to validate these findings.
Acknowledgments
Authors would like to thank all the enrolled patients and their caregivers without whom the study would not have been possible.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Sajan K. Hegde: senior consultant, main operating surgeon; Vigneshwara Badikillaya: consultant, operating surgeon; Umesh P. Kanade: assist fellow, data compilation, manuscript formation; Keyur Akbari: assist fellow, data & manuscript analysis; T. Sharan Achar: assist fellow, data compilation, manuscript formatting; B. Harith Reddy: senior registrar.