 |
 |
- Search
| Asian Spine J > Volume 17(6); 2023 > Article |
|
Abstract
Purpose
This study aimed to determine whether the initiation of anti-calcitonin gene-related peptide (CGRP inhibitor) medication therapy for migraines was also associated with improvements in back/neck pain, mobility, and function in a patient population with comorbid degenerative spinal disease and migraine.
Overview of Literature
CGRP upregulates pro-inflammatory cytokines such as tumor necrosis factor-╬▒, interleukin-6, brain-derived neurotrophic factor, and nerve growth factor in spinal spondylotic disease, which results in disc degeneration and sensitization of nociceptive neurons. Although CGRP inhibitors can quell neurogenic inflammation in migraines, their off-site efficacy as a therapeutic target for discogenic back/neck pain conditions remains unknown.
Methods
All adult patients diagnosed with spinal spondylosis and migraine treated with CGRP inhibitors at a single academic institution between 2017 and 2020 were retrospectively identified. Patient demographic and medical data, follow-up duration, migraine severity and frequency, spinal pain, functional status, and mobility before and after the administration of CGRP inhibitors were collected. Paired univariate analysis was conducted to determine significant changes in spinal pain, headache severity, and headache frequency before and after the administration of CGRP inhibitors. The correlation between changes in the spinal pain score and functional or mobility improvement was assessed with SpearmanŌĆÖs rho.
Results
In total, 56 patients were included. The mean follow-up time after the administration of CGRP inhibitors was 123 days for spinal pain visits and 129 days for migraine visits. Back/neck pain decreased significantly (p<0.001) from 6.30 to 4.36 after starting CGRP inhibitor therapy for migraine control. As recorded in the spine follow-up notes, 25% of patients experienced a functional improvement in the activities of daily living, and 17.5% experienced mobility improvement while taking CGRP inhibitors. Change in back/neck pain moderately correlated (Žü=ŌłÆ0.430) with functional improvement but was not correlated with mobility improvement (Žü=ŌłÆ0.052).
Back and neck pain are leading causes of medical burden, economic cost, and disability in the United States, with a lifetime prevalence of 60%ŌĆō80% and 20%ŌĆō70%, respectively [1]. Intervertebral disc (IVD) degeneration has been implicated in back/neck pain and is believed to have multifactorial etiology. The decline in nutrient supply, altered extracellular matrix composition, endplate calcification, mechanical damage, and genetics have all been associated with IVD degeneration [1,2]. Despite the immense burden of spinal degenerative diseases, current treatment options to address discogenic pain including oral analgesic therapy, physical therapy, acupuncture, and corticosteroid injections have demonstrated minimal efficacy [1,3]. Surgical interventions for degenerative disc conditions associated with back pain rarely result in the full resolution of back/neck pain [4]. Accordingly, no current standard has been established for treating discogenic spinal pain that predictably results in clinical improvement [1,5].
Studies have investigated conservative therapies aimed at disrupting the natural history of discogenic pain. Approaches targeting IVD regeneration including stem cell-based therapy preliminarily showed improvements in discogenic lower back pain in animal models and human trials [6]. Moreover, emerging research to characterize cytokines and inflammatory mediators contributing to discogenic degeneration has elucidated additional targets for novel therapeutics. Specifically, cytokines such tumor necrosis factor-alpha (TNF-╬▒) and interleukin-1 beta (IL-1╬▓) and the pro-inflammatory neuropeptide substance P (SP) have been observed to contribute to inflammation at the level of IVD cells [7,8]. However, early clinical trials aimed at inflammatory mediator blockade involving pathways with these targets have produced inconclusive results [9,10]. In 2017, the Food & Drug Administration (FDA) approved a new class of biologic injectable monoclonal antibodies that target the inhibition of calcitonin gene-related peptide (CGRP)-associated neurogenic inflammation for the treatment of episodic or chronic migraine headaches [11]. CGRP is found in abundance at the interface between blood vessels and unmyelinated (C) and thinly myelinated (A╬┤) sensory nerve fibers and upregulates pro-inflammatory mediators such as TNF-╬▒, IL-6, brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF) in IVD cells resulting in further IVD degeneration and sensitization of nociceptive neurons (Fig. 1) [11,12].
The IVD stores of CGRP suggest that it may be significantly involved in IVD degeneration and symptoms of discogenic back pain. CGRP inhibitors are currently approved by the FDA for use only in intractable migraine [13]. Accordingly, this study investigated the potential therapeutic off-target effects on the spine using a patient population with comorbid degenerative spinal diseases and migraines managed with CGRP inhibitors. Thus, this study aimed to determine whether the initiation of CGRP inhibitor therapy for migraine was also associated with improvements in back/neck pain, mobility, and function.
After the approval by the institutional review board of Thomas Jefferson University Hospital (IRB no., 20E.085), all patients aged >18 years with concomitant spinal degenerative conditions and migraine at a single academic institution between 2017 and 2020 were retrospectively identified. The IRB approval waived the need for patient consent as the study was a minimal-risk non-interventional study. All patients had evidence of spinal spondylosis or IVD of the cervical, thoracic, or lumbar spine and were identified according to the International Classification of Diseases 10th revision (ICD-10) codes (Supplement 1). Patients were included based on the presence of comorbid migraine diagnosed by a neurologist and treated with CGRP inhibitors, including erenumab, fremanzumab, galcanezumab, and ubrogepant (Supplement 1). Additional inclusion criteria required complete patient demographic profiles, medication lists, neurology and spinal encounter notes, and quantitative metrics of the spinal and migraine severity or frequency before and after the initiation of CGRP inhibitors. All patients with cervicogenic headache, previous (within 5 years before the study period) or active malignancy, or those with the initiation of or increased dosing of opioid medications over the study interval were excluded. The spinal exclusion criteria were asymptomatic incidentally detected degenerative spinal conditions without clinical follow-up and transient back/neck pain managed exclusively in the primary care setting or any history of spinal surgery before or during the study period. Patients were also excluded if CGRP inhibitors were not administered to control their migraines or if there was evidence of gaps in receiving CGRP inhibitors according to prescription records.
Patient demographic and medical data including age, sex, race, smoking status (never, former, and current), body mass index (BMI), Charlson comorbidity index (CCI), history of depression, anxiety, or fibromyalgia, and duration of the clinical follow-up of the spine and migraine following the initiation of CGRP inhibitor therapy were obtained via chart review. The primary results were back/neck pain scores, measured on a 0ŌĆō10 scale, before and after the initiation of CGRP inhibitors. Pain scores were obtained from the institutionŌĆÖs recording software (Epic Systems, Verona, WI, USA). Migraine severity (0ŌĆō10 scale) and frequency of symptoms (days per week) were extracted from neurology encounter notes. In cases where patients attended multiple spine- and migraine-related clinical appointments after the initiation of CGRP inhibitors, the post-scores were extracted from the greatest possible follow-up duration. Individual patient delta (╬ö) outcome scores were calculated by subtracting their pre-therapy score from the post-therapy score, with therapy being the initiation of CGRP inhibitors.
Functional and mobility status was determined at each spine visit from chart review. The degree of impairment in activities of daily living (ADLs) was assessed through the same metrics as the physical component score questionnaires. Mobility was evaluated through the Physical Mobility Scale (PMS) based on patient history or physical examinations. Improvement in function and mobility between spine clinical visits was determined by decreased impairment in one or more ADLs and one or more point increases in PMS, respectively.
Paired univariate analysis was conducted to determine significant changes in spinal pain, migraine severity, and migraine frequency before and after the initiation of the CGRP inhibitor therapy. The correlation between the ╬ö spinal pain score and functional or mobility improvement were assessed using SpearmanŌĆÖs rho (Žü) coefficient. SpearmanŌĆÖs rho was interpreted as follows: 0ŌĆō0.20 as negligible correlation; 0.21ŌĆō0.40, weak correlation; 0.41ŌĆō0.60, moderate correlation; 0.61ŌĆō0.80, strong correlation; and 0.81ŌĆō1.00, very strong correlation. All statistical analysis was performed with the RStudio ver. 4.0.2 (RStudio, Boston, MA, USA). A p-value <0.05 was considered statistically significant.
Patient identification according to inclusion/exclusion criteria is presented in Fig. 2. A total of 56 patients were included. The mean follow-up times following initiation of CGRP inhibitor therapy were 123 and 129 days for spinal and migraine visits, respectively. The mean patient age was 55 years, and 85.7% were women and 14.3% were men of Caucasian (82.1%), African American (12.5%), and other (5.4%) descents (Table 1). Regarding smoking status, 51.8%, 8.9%, and 39.3% of the patients were nonsmokers, current smokers, and former smokers, respectively. The mean BMI and CCI were 28.3 and 1.2, respectively. In the overall cohort, the incidence rates of depression, anxiety, and fibromyalgia were 41.1%, 51.8%, and 41.1%, respectively (Table 1).
With regard to spinal visits, back/neck pain decreased significantly (p<0.001) from 6.30 to 4.36 after starting CGRP inhibitor therapy for migraine control (Table 2). As recorded in the spine follow-up notes, 25% of the patients experienced functional improvement in ADLs and 17.5% experienced mobility improvement while taking CGRP inhibitors (Table 3). A change in back/neck pain demonstrated a moderate correlation (Žü=ŌłÆ0.430) with functional improvement. However, changes in spinal pain scores were not correlated (Žü=ŌłÆ0.052) with improvements in mobility (Table 3). Regarding clinical neurology visits, migraine severity (pre with 6.34 versus post with 5.42, p=0.007) and frequency (pre with 5.16 versus post with 3.31, p=0.004) decreased significantly following the initiation of CGRP inhibitor therapy (Table 4).
CGRP inhibitors have proven efficacy for the acute and preventative treatment of migraine through the mitigation of neurogenic inflammation; however, their off-site efficacy as a therapeutic target for degenerative back and neck pain remains unknown [14]. Accordingly, this study sought to determine whether patients with spinal spondylosis and concurrent migraine diagnosis experience improvement in their back/neck pain and functional level upon the initiation of CGRP inhibitor therapy. The results of this study suggest that the initiation of CGRP inhibitors resulted in significant back/neck pain reduction in patients with degenerative spinal conditions.
CGRP is a 37-amino acid neuropeptide that binds to a unique cell surface heterodimeric receptor composed of receptor activity modifying protein one and calcitonin receptor-like receptor to mediate nociception and somatic pain pathways in the central and peripheral nervous systems [11,12]. CGRP and its receptors are abundant perivascularly and act via cyclic adenosine monophosphate-triggered vasodilatory pathways to precipitate endothelium-derived nitric oxide (NO) smooth muscle relaxation, vascular permeability, and inflammatory infiltration [11,12]. Furthermore, CGRP is a critical mediator of spinal inflammatory pathways to perpetuate neurogenic inflammation and persistent nociception [11,15]. In this study, CGRP inhibitors act as monoclonal antibodies to directly antagonize the CGRP ligand or receptor [14,16].
In spinal spondylotic disease, discogenic cells (nucleus pulposus and annulus fibrosis), neutrophils, macrophages, and T cells produce high levels of pro-inflammatory molecules, including IL-1 ╬▒/╬▓, IL-2, IL-4, IL-6, IL-8, IL-10, IL-17, TNF-╬▒, IFN-╬│, chemokines, and prostaglandin E2 (PGE2), to mediate matrix degradation [17]. The structural defects in the extracellular matrix increase the risk of disc herniation and can lead to a feedback cycle of increased immune cell activation/infiltration, nerve irritation, and neovascular and nociceptive fiber growth arising from the dorsal root ganglion (DRG) that can potentiate pain signals to the IVD through the sinuvertebral nerves [17]. In this inflammatory milieu, infiltrating cells release neurotrophins NGF and BDNF, which are associated with pain-generating cationic channels at the DRG level [17]. CGRP is intimately linked with the local release of NGF and BDNF and is upregulated in models of nerve damage and inflammatory change (Fig. 1) [18]. Evidence suggests that CGRP may be coproduced with the tachykinin SP, a known paracrine and autocrine mediator of degenerative disc disease (DDD), leading to the upregulation of IL-1╬▓, IL-6, and IL-8 in the nucleus pulposus and annulus fibrosis cells [19]. Additionally, local delivery of TNF-╬▒ inhibitors in a rat DDD model suppressed CGRP immunoreactivity in DRG neurons, underscoring the interrelationship between IVD degeneration and CGRP neuronal activation [20]. Therefore, the antagonism of the CGRP ligand or receptor was postulated to suppress inflammatory cytokine and neurotrophin response implicated in the aforementioned pathogenesis of DDD and associated pain.
Despite the persistent need for novel treatments for discogenic back/neck pain, previous clinical trials to therapeutically inhibit known spinal inflammatory mediators have obtained mixed results that have yet to change treatment approaches. In cellular models of DDD, antagonism of various inflammatory cytokines, neurotrophins, and tachykinins successfully attenuated IL and matrix metalloproteases (MMT) expression [21,22]. Numerous studies have reported that the coincubation of disc cells with TNF-╬▒, IL-1╬▓, IL-17, or SP in the presence of their corresponding antagonists demonstrated significant reductions in NO, PGE2, cycloxgenase-2, IL-1╬▓, IL-6, IL-8, and/or MMT compared with incubation with the particular inflammatory mediator alone [22]. The successful mitigation of discogenic degeneration attributed to inflammatory marker inhibitors in vitro directed clinical trials to determine the effectiveness of monoclonal antibodies antagonizing TNF-╬▒, NGF, and IL-6 for lower back pain [23]. Investigations of TNF-╬▒ inhibitors, including intravenous infliximab and epidural etanercept therapies, for the treatment of sciatica revealed statistically significant pain reduction at 1ŌĆō3 months and increased return to work at 3 months compared with controls [24]. However, when intradiscal etanercept therapy was initiated in the treatment of discogenic back pain and lumbosacral radiculopathy, no differences in pain or disability scores were identified between the treatment and placebo groups at the 1-month follow-up [25]. In several trials exploring the intravenous administration of tanezumab, as NGF inhibitor, to treat chronic lower back pain found that decreased pain intensity and disability after 6 and 18 weeks compared with naproxen and placebo [26]. This class of medications has been largely abandoned because of safety concerns where paresthesias, arthralgias, and osteonecrosis have been associated with high tanezumab doses [27]. Recent investigations have demonstrated significantly an increased risk of rapidly progressive osteoarthritis that often requires total joint arthroplasty in the tanezumab versus placebo groups [28,29]. Finally, the intradiscal injection of IL-6 receptor antibody, tocilizumab, for discogenic back pain demonstrated significant reductions in pain and disability index scores at 2 and 4 weeks relative to control [9]. To address neurogenic inflammation in DDD, the short-term analgesic effects exhibited in select trials of inflammatory mediator antagonists inspire the investigation of novel off-target applications of CGRP inhibitors.
This retrospective analysis utilizes a readily available patient population that received CGRP inhibitors for migraine control and had a concomitant degenerative spinal diagnosis. In this small patient cohort, the initiation of CGRP inhibitors led to a significant reduction in back/neck pain that may be attributed to the off-site efficacy of CGRP blockade to reduce cytokine production, inflammatory infiltration, and neovascularization and neoneuralization at the level of the spinal disc and DRG. Furthermore, the correlation between decreased spinal pain and increased function suggests that patients who benefit from CGRP inhibition in the spine are more likely to experience improvement in the quality of life. On average, the analyzed patients also experienced significant reductions in migraine severity and frequency, demonstrating the expected response to the on-label medication usage previously associated with >50% reduction in monthly migraines in 50% of the patients [30]. More studies on patients with both migraine and degenerative spinal conditions should attempt to identify whether hyper-responders to CGRP inhibitors also experience the greatest improvement in back/neck pain. Moreover, the findings of this retrospective cohort study necessitate further exploration in prospective and interventional studies to determine the effectiveness of CGRP blockade in all patients with DDD.
This study has limitations, including those inherent to a retrospective cohort study. The narrow inclusion criteria of patients currently taking CGRP inhibitors and receiving nonoperative care for spinal spondylosis at a single institution represent only a subset of individuals that could benefit from novel biologic therapies for DDD. The small cohort size and variations in the frequency of patient follow-up did not enable analyses regarding the rate of back/neck pain reduction or the relationship between improvement in spinal and migraine conditions. Although patients were systematically enrolled based on the ICD-10 codes corresponding to spinal spondylosis with or without instability or stenosis, spinal diagnostic imaging was unavailable for all patients, and the cohort size limited the stratified outcome analysis based on the spinal diagnosis. Given that patients undergoing spinal surgery were excluded, the potential effects of nonoperative treatment modalities on back/neck pain remain uncontrolled for in this study. Furthermore, the lack of consistent patient outcome assessment at the spinal follow-up limits the quantification of functional and disability improvement upon the initiation of CGRP inhibitor therapy. Finally, prescription records may only approximate patient adherence to CGRP inhibitor therapy.
Patients on CGRP inhibitor therapy for the treatment of intractable migraine with comorbid degenerative spinal conditions experienced significant off-target back/neck pain reduction. Future interventional studies must explore the effectiveness of this novel class of medications in all patients with discogenic back/neck pain that is recalcitrant to standard nonoperative therapies.
Notes
Author Contributions
Conceptualization: JAC, HAL, MC, CKK, DZM, BAK; data curation: HAL, OB, MC, NP, JC; formal analysis: HAL, JAC, OB, MC, NP, JC; funding acquisition: JAC, CKK, GDS, ARV, DZM; methodology: JAC, HAL, CKK, DZM; project administration: JAC, CKK, DZM, BAK; visualization: HAL, MC, BAK; writingŌĆōoriginal draft: HAL, MC, BAK, OB, NP, JC; writingŌĆōreview & editing: JAC, HAL, BAK, ASH, GDS, ARV, DZM, DES, CKK; and final approval of the manuscript: all authors.
Supplementary Materials
Supplementary materials can be available from https://doi.org/10.31616/2023.0121.
Supplement 1.
Supplemental content: ICD-10 codes for patient inclusion criteria.
Fig.┬Ā1
Calcitonin gene-related peptide (CGRP) inflammatory cascade in the degenerating disc. IL-6, interleukin-6; TNF-╬▒, tumor necrosis factor-alpha; BDNF, brain-derived neurotrophic factor; NGF, nerve growth factor.
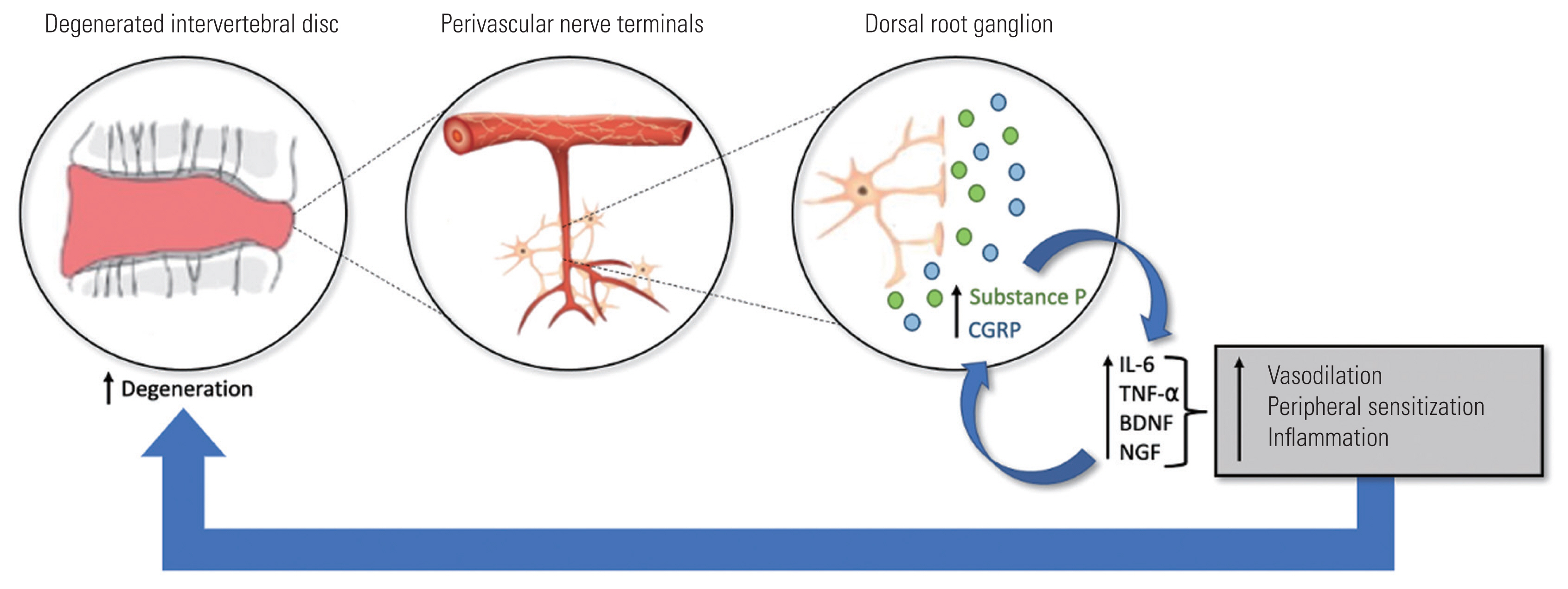
Fig.┬Ā2
Patient cohort generation according to inclusion/exclusion criteria. CGRP, calcitonin gene-related peptide.

Table┬Ā1
Patient cohort demographics with degenerative spinal disease and migraine diagnoses
Table┬Ā2
Discogenic spinal pain before vs after anti-calcitonin gene-related peptide medication initiation (N=33)
| Spinal parameter | Mean value | p-value |
|---|---|---|
| Pre pain score | 6.30 | <0.001* |
| Post pain score | 4.36 | |
| ╬ö Pain Score | ŌłÆ1.94 |
Table┬Ā3
Change in spinal symptoms before vs. after anti-calcitonin gene-related peptide medication initiation (N=29)
| Spinal symptoms | % | Correlation with ╬ö pain score (Žü) |
|---|---|---|
| Functional ADLs | ŌłÆ0.430a) | |
| ŌĆāImprovement | 25.0 | |
| ŌĆāNo improvement | 75.0 | |
| Mobility | ŌłÆ0.052 | |
| ŌĆāImprovement | 17.5 | |
| ŌĆāNo improvement | 82.5 |
References
1. Fujii K, Yamazaki M, Kang JD, et al. Discogenic back pain: literature review of definition, diagnosis, and treatment. JBMR Plus 2019;3:e10180.




2. Dowdell J, Erwin M, Choma T, Vaccaro A, Iatridis J, Cho SK. Intervertebral disk degeneration and repair. Neurosurgery 2017;80:S46ŌĆō54.



3. Yang AJ, Coronado RA, Hoffecker L, et al. Conservative care in lumbar spine surgery trials: a descriptive literature review. Arch Phys Med Rehabil 2017;98:165ŌĆō72.


4. Parker SL, Mendenhall SK, Godil SS, et al. Incidence of low back pain after lumbar discectomy for herniated disc and its effect on patient-reported outcomes. Clin Orthop Relat Res 2015;473:1988ŌĆō99.



5. Amirdelfan K, Webster L, Poree L, Sukul V, McRoberts P. Treatment options for failed back surgery syndrome patients with refractory chronic pain: an evidence based approach. Spine (Phila Pa 1976) 2017;42(Suppl 14): S41ŌĆō52.


6. Urits I, Capuco A, Sharma M, et al. Stem cell therapies for treatment of discogenic low back pain: a comprehensive review. Curr Pain Headache Rep 2019;23:65.



7. Kepler CK, Markova DZ, Hilibrand AS, et al. Substance P stimulates production of inflammatory cytokines in human disc cells. Spine (Phila Pa 1976) 2013;38:E1291ŌĆō9.


8. Koerner JD, Markova DZ, Schroeder GD, et al. The effect of substance P on an intervertebral disc rat organ culture model. Spine (Phila Pa 1976) 2016;41:1851ŌĆō9.


9. Sainoh T, Orita S, Miyagi M, et al. Single intradiscal injection of the interleukin-6 receptor antibody tocilizumab provides short-term relief of discogenic low back pain; prospective comparative cohort study. J Orthop Sci 2016;21:2ŌĆō6.


10. Cohen SP, Bogduk N, Dragovich A, et al. Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology 2009;110:1116ŌĆō26.



11. Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 2014;94:1099ŌĆō142.



12. Schou WS, Ashina S, Amin FM, Goadsby PJ, Ashina M. Calcitonin gene-related peptide and pain: a systematic review. J Headache Pain 2017;18:34.




13. Krock E, Rosenzweig DH, Chabot-Dore AJ, et al. Painful, degenerating intervertebral discs up-regulate neurite sprouting and CGRP through nociceptive factors. J Cell Mol Med 2014;18:1213ŌĆō25.


14. Raffaelli B, Reuter U. The biology of monoclonal antibodies: focus on calcitonin gene-related peptide for prophylactic migraine therapy. Neurotherapeutics 2018;15:324ŌĆō35.




15. Brain SD, Geppetti P. Calcitonin gene-related peptide (CGRP) mechanisms: focus on migraine. Cham: Springer; 2019.
17. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 2014;10:44ŌĆō56.




18. Orita S, Eguchi Y, Kamoda H, et al. Brain-derived neurotrophic factor inhibition at the punctured intervertebral disc downregulates the production of calcitonin gene-related peptide in dorsal root ganglia in rats. Spine (Phila Pa 1976) 2011;36:1737ŌĆō43.


19. Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Substance P and calcitonin gene-related peptide immunoreactive sensory DRG neurons innervating the lumbar intervertebral discs in rats. Ann Anat 2002;184:235ŌĆō40.


20. Horii M, Orita S, Nagata M, et al. Direct application of the tumor necrosis factor-╬▒ inhibitor, etanercept, into a punctured intervertebral disc decreases calcitonin gene-related peptide expression in rat dorsal root ganglion neurons. Spine (Phila Pa 1976) 2011;36:E80ŌĆō5.


21. Sinclair SM, Shamji MF, Chen J, et al. Attenuation of inflammatory events in human intervertebral disc cells with a tumor necrosis factor antagonist. Spine (Phila Pa 1976) 2011;36:1190ŌĆō6.



22. Kepler CK, Markova DZ, Dibra F, et al. Expression and relationship of proinflammatory chemokine RANTES/CCL5 and cytokine IL-1╬▓ in painful human intervertebral discs. Spine (Phila Pa 1976) 2013;38:873ŌĆō80.


23. Lyu FJ, Cui H, Pan H, et al. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res 2021;9:7.




24. Cohen SP, Bogduk N, Dragovich A, et al. Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology 2009;110:1116ŌĆō26.



25. Cohen SP, Wenzell D, Hurley RW, et al. A double-blind, placebo-controlled, dose-response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology 2007;107:99ŌĆō105.



26. Kivitz AJ, Gimbel JS, Bramson C, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 2013;154:1009ŌĆō21.


27. Hochberg MC, Tive LA, Abramson SB, et al. When is osteonecrosis not osteonecrosis?: adjudication of reported serious adverse joint events in the tanezumab clinical development program. Arthritis Rheumatol 2016;68:382ŌĆō91.


28. Markman JD, Bolash RB, McAlindon TE, et al. Tanezumab for chronic low back pain: a randomized, double-blind, placebo- and active-controlled, phase 3 study of efficacy and safety. Pain 2020;161:2068ŌĆō78.










