 |
 |
- Search
| Asian Spine J > Volume 18(2); 2024 > Article |
|
Abstract
Purpose
This study aimed to understand the role of magnetic resonance imaging (MRI) in predicting neurological deficits in traumatic lower lumbar fractures (LLFs; L3–L5).
Overview of Literature
Despite studies on the radiological risk factors for neurological deficits in thoracolumbar fractures, very few have focused on LLFs. Moreover, the potential utility of MRI in LLFs has not been evaluated.
Methods
In total, 108 patients who underwent surgery for traumatic LLFs between January 2010 and January 2020 were reviewed to obtain their demographic details, injury level, and neurology status at the time of presentation (American Spinal Injury Association [ASIA] grade). Preoperative computed tomography scans were used to measure parameters such as anterior vertebral body height, posterior vertebral body height, loss of vertebral body height, local kyphosis, retropulsion of fracture fragment, interpedicular distance, canal compromise, sagittal transverse ratio, and presence of vertical lamina fracture. MRI was used to measure the canal encroachment ratio (CER), cross-sectional area of the thecal sac (CSAT), and presence of an epidural hematoma.
Results
Of the 108 patients, 9 (8.3%) had ASIA A, 4 (3.7%) had ASIA B, 17 (15.7%) had ASIA C, 21 (19.4%) had ASIA D, and 57 (52.9%) had ASIA E neurology upon admission. The Thoracolumbar Injury Classification and Severity score (p=0.000), CER (p=0.050), and CSAT (p=0.019) were found to be independently associated with neurological deficits on the multivariate analysis. The receiver operating characteristic curves showed that only CER (area under the curve [AUC], 0.926; 95% confidence interval [CI], 0.860–0.968) and CSAT (AUC, 0.963; 95% CI, 0.908–0.990) had good discriminatory ability, with the optimal cutoff of 50% and 65.3 mm2, respectively.
Traumatic fractures of the low lumbar spine (L3–L5) are uncommon, accounting for only 10%–13% of thoracolumbar (TL) injuries [1,2]. In comparison to the thoracic and TL spinal regions, the lower lumbar spine has unique anatomical and biomechanical characteristics [3,4]. The lordotic position of the lower lumbar spine shifts the body’s center of gravity posterior to the vertebral body, resulting in an even distribution of axial compression force and higher risks of burst fractures [5].
As the spinal canal in the lower lumbar region is comparatively wider, injuries to the cauda equina and spinal root, although result in neurological impairment, have a higher chance of recovery following surgery [5–8]. Numerous studies have investigated radiological findings related to neurological deficits in TL fractures, such as the canal anteroposterior diameter, anterior vertebral compression ratio, interpedicular distance (IPD), and presence of vertical laminar fractures [9–14]. In a study of 198 patients with TL and lumbar fractures, Meves and Avanzi [15] reported a significant correlation between spinal canal stenosis and the severity of incomplete neurologic deficits. However, most studies have analyzed computed tomography (CT)-based parameters and focused on TL fractures [15].
Recently, Lee et al. [16] analyzed various CT parameters to determine the incidence of neurological deficit in patients with mid and lower lumbar fractures (LLFs, L2–L5). However, their results were constrained by the relatively small sample size and lack of magnetic resonance imaging (MRI) parameters [16]. Moreover, the utility of MRI in predicting neurological deficits in cervical and thoracic spine injuries has been widely studied based on cord signal changes. Its applicability in lumbar spine fractures has been least explored. Thus, we decided to analyze both CT and MRI parameters in our patients with LLFs because of some cases of spinal epidural hematoma formation in LLFs that resulted in neurological deficits.
Between January 2010 and January 2020, data from 108 patients with traumatic LLFs who underwent surgical management in Ganga Medical Center and Hospitals Pvt. Ltd. (Ganga Hospital) were retrospectively reviewed after clearance from the institutional review board (IRB no., 2022/10/12). On the contrary, patients who had inadequate follow-up data, lacked CT/MRI data, had undergone spine surgery, and had pathological fractures were excluded. The requirement for informed consent from individual patients was omitted because of the retrospective design of this study.
Patient demographic details were collected from both the medical records and the hospital information system. The assessment of the neurological status was documented using the American Spinal Injury Association (ASIA) Impairment scale. Patients were classified into two groups, namely, patients with neurodeficits (ASIA A, B, C, and D) and patients with normal neurology (ASIA E), and radiological predictors were analyzed between the two groups. Fractures were categorized by the Thoracolumbar Injury Classification and Severity (TLICS) score, load-sharing classification score, and AO spine classification.
In all patients, CT images were focused on the bone tissue with a mean window width of 2,000 Hounsfield units and a mean window level of 500 Hounsfield units and axial images of 3-mm slice thickness, and sagittal and coronal images were reconstructed with a slice thickness of 2 mm. MRI was performed using a 1.5-T MRI (Magnetom Symphony; Siemens, Erlangen, Germany). The spine trauma protocol included axial and sagittal T2- and T1-weighted, sagittal short tau inversion recovery, with a slice thickness of 3 mm for sagittal and 4 mm for axial, using a matrix size of 240×320.
The following radiological parameters were analyzed in preoperative CT images.
This was measured as the distance between the most anterior superior point to the most anterior inferior point on the vertebral body rim (Fig. 1).
This was measured as the distance between the most posterior superior point to the most posterior inferior point on the vertebral body rim (Fig. 1).
The affected anterior and posterior vertebral body heights of the segment were measured. A normal loss of vertebral body height (LOVBH) was determined as a percentage of the height loss normalized to the average of the vertebral bodies above and below the injured segment, as previously explained by Willen et al. [17] (Fig. 1).
Local kyphosis (LK) refers to the angle formed between the lines drawn along the superior and inferior endplates of the fractured vertebra (Fig. 1).
Retropulsion of the fracture fragment (RFF) refers to the distance from the posterosuperior corner of the fractured vertebral body to a tangent line touching the posterior inferior corner of the above normal vertebra (Fig. 1).
IPD was measured in axial CT cuts by comparing the distance between the medial borders of both pedicles at the injury level and the normal levels above (PA) and below (PB) the injury level (Fig. 2).
Canal compromise (CC) was measured as the cross-sectional area of the spinal canal at the fracture level in axial CT by drawing the region of interest (ROI) on the Picture Archiving and Communication System (PACS), which automatically calculated the cross-sectional area (Fig. 2).
The sagittal transverse ratio (STR) was defined as the ratio of the anteroposterior spinal canal diameter to the medial–lateral spinal canal diameter (Fig. 2).
The following radiological parameters were analyzed using preoperative MRI scans.
Canal encroachment ratio (CER) was calculated using the anteroposterior canal diameter of the fractured vertebra (DI) and the vertebrae above (DA) and below (DB) on the midsagittal MRI (Fig. 3).
This was measured on the selected axial image by drawing an ROI around the thecal sac on a PACS, which then automatically calculated the cross-sectional area of the thecal sac (CSAT) (Fig 3).
This was diagnosed based on the isointense or hyperintense signal present on T1W and hyperintensity with areas of hypointensity to the spinal cord in T2W images causing compression of epidural fat, subarachnoid sac, and spinal cord (Fig. 4).
Statistical analysis was performed using IBM SPSS Statistics for Windows ver. 24.0 (IBM Corp., Armonk, NY, USA). The unpaired t-test was used to analyze continuous variables, whereas the chi-square test or Fisher’s exact test was used to analyze categorical ones. Wilson confidence intervals (CIs) were used to determine CIs for proportions. Multivariate logistic regression analysis was used to determine the independent risk variables associated with neurological deficits. Variables significant at p<0.05 in the univariate analyses were eligible for inclusion in the multivariate models. It determined the diagnostic accuracy of radiological parameters for predicting neurological deficit, namely, sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios. To determine the cutoff, receiver operating characteristic (ROC) curves were generated. Using the area under the curve (AUC), the discriminatory ability of risk factors was evaluated. The threshold was determined based on the Youden index.
Of the 108 patients, 96 were males and 12 were females. The mean age was 35.8 years (range, 15–84 years). Moreover, 71 patients (65.7%) had L3 fracture, 34 (31.5%) had L4 fracture, and 3 (2.8%) had L5 fracture. MRI identified posterior ligamentous complex (PLC) injury in 57 patients (52.8%). Among the 108 patients, 9 (8.3%) had ASIA A, 4 (3.7%) had ASIA B, 17 (15.7%) had ASIA C, 21 (19.4%) had ASIA D, and 57 (52.9%) had ASIA E neurology status upon admission. Epidural hematoma was noted significantly higher (p-value=0.001) in the group with neurodeficit (64.7%) than in the group without neurodeficit (7%) (Table 1).
The neurodeficit group had a substantially higher mean TLICS (7.08±1.07) than the control group (4.58±0.75) (p=0.001). Furthermore, the mean load-sharing score for the neurodeficit group was 7.82±1.29, which was substantially higher (p=0.001) than that for the control group, which was 6.67±1.95. Moreover, statistically significant differences in RFF (p=0.001), CC (p=0.001), CER (p=0.001), and CSAT (p=0.001) were found between the groups (Table 2).
The TLICS, load-sharing score, RFF, CC, CER, CSAT, and presence of epidural hematoma were found significant (p<0.05) on the univariate analysis (Table 3). However, multivariate logistic regression analysis revealed that TLICS (odds ratio, 19.124; 95% CI, 4.647–78.699; p=0.000), CER (odds ratio, 1.062; 95% CI, 1.000–1.128; p=0.050), and CSAT (odds ratio, 0.924; 95% CI, 0.865–0.987; p=0.019) were independently associated with neurological deficit (Table 4). Only CER and CSAT have high discriminating power, as shown by ROC analyses (AUC, 0.926; 95% CI, 0.860–0.968; AUC, 0.963; 95% CI, 0.908–0.990, respectively). The optimal cutoff value for CER was 50% with a sensitivity and specificity of 84.3% and 91.2%, respectively. Similarly, the optimal cutoff for CSAT was 65.3 mm2 with a sensitivity and specificity of 94.7% and 90.2%, respectively.
The results of our study highlight that among CT and MRI parameters, the major determinants of neurological deficits in traumatic LLFs are the CER and CSAT. Both are MRI-based parameters, implicating the importance of MRI in patients with LLFs.
MRI has been the imaging modality of choice in acute spinal injury. To evaluate spinal cord damage and rule out soft tissue injuries, epidural hematoma, or disc herniation, MRI has largely replaced CT as the modality of choice, even if CT can reveal more information about the extent of a bone injury [10]. Various qualitative and quantitative parameters are used to assess and prognosticate the extent of spinal cord injury (SCI) [9–15]. Ramon et al. [18] classified qualitative MRI parameters into five patterns: pattern 1, hemorrhage; pattern 2, edema; pattern 3, contusion; pattern 4, compression; and pattern 5, transection. They highlighted that patients with cord hemorrhage and transection were associated with irreversible neurological deficits, whereas those with contusion and edema were associated with reversible neurological deficits [18].
Similarly, the quantitative parameters used for assessing SCI are maximum canal compromise (MCC) and maximum spinal cord compression (MSCC), which reflect acute neurological deficits caused by cord compression. The optimum MCC and MSCC cutoffs are 29% and 52.8% in the cervical region and 30% and 24.0% in the cervicothoracic region, respectively [19,20]. These MRI parameters have been widely used in patients with acute cervical and thoracic SCIs.
Biomechanically, the TL region (T11–L1) represents a transitional region between the rigid thoracic and mobile lumbar spine. The neurological deficit pattern at T11 and T12 differs significantly from that of L1. This may be attributed to the variation in the position of the conus medullaris, variable involvement, and sensitivity of the cord, conus, or cauda equina at the level of injury. Similarly, the mid (L2) and lower lumbar (L3–L5) regions have wider spinal canal diameter; thus, injuries to the lower motor neuron, such as the cauda equina and spinal root, cause neurological impairment but have a high chance of recovery following surgery than injuries to the upper motor neurons [5–8].
Several studies have reported radiographic findings that can predict neurological deficits in TL fractures, including spinal canal stenosis, vertebral body height loss, IPD, and presence of vertical laminar fractures [11,12]. According to Hashimoto et al. [21], the incidence of neurological deficit in patients with burst fractures was significantly high when the degree of CC was ≥35% at T11 and T12, ≥45% at L1, and ≥55% in other lumbar vertebrae. Meanwhile, Mohanty et al. [13] found a median of 50% spinal CC in their group of patients with neurological deficits caused by TL fractures, they were unable to find a statistically significant relationship between CC and severity of neurological deficit. The majority of radiological parameters described in the literature are based on CT, whereas MRI results are just briefly mentioned. In a study of 76 patients with acute TL fractures, Naduvanahalli Vivekanandaswamy et al. [22] found that among all MRI-based parameters, the length of cord edema was the most significant predictor of neurological deficits.
The radiological predictors of neurological deficit in LLFs may vary substantially from those in TL fractures. Lehman et al. [23] reported that in 19 patients with LLFs, 10 (52.6%) had neurological deficits. Similarly, Motten et al. [24] reported an incidence rate of 33% in their patients with LLFs. In the present study, out of 108 patients, 51 (47.2%) had neurological deficits, which was comparable to previous reports.
In TL fractures, neurological impairment is associated with the fracture type, injury level, kyphotic deformity, and various parameters [13,25,26]. The results of this study showed that the injury level and type did not correlate with the incidence of neurological deficits. Moreover, no correlation was found between the incidence of neurological deficit and the presence of lamina fractures or PLC injury. Among CT parameters, RFF and CC were found to be significantly higher in patients with neurological deficits. However, other parameters such as the LOVBH, LK, IPD, and STR were not significant.
In this study, MRI parameters, namely CER, CSAT, and presence of epidural hematoma, were analyzed. The importance of a critical analysis of these parameters can be substantiated by our results. On the multivariate analysis of all radiological parameters, only CER and CSAT were statistically significant.
Although the incidence of neurological deficit is as high as 60% in patients with burst fractures, the degree of CC may vary [27]. According to Denis [28], the obstruction of 25%–50% of the spinal canal rarely resulted in neurological damage at the L2–L5 level, although it was comparatively higher at the T11–L1 level. Hashimoto et al. [21] reported that the optimal cutoff of CER was 55% at the level of cauda equina in 22 patients with LLFs. In a study involving 71 patients with mid and LLFs, Lee et al. [16] reported a cutoff of 47% as the optimal value for patients to undergo emergency decompression.
According to Sandler and Tator [29], the degree of neurological damage is primarily determined by the severity of the initial insult, and the deficit may continue despite the elimination of the spinal cord pressure. Moreover, during injury, various dynamic changes in the geometry of the spinal canal, vertebral endplates, and intervertebral disc result in a higher magnitude of damage to the neural structures in the canal [29]. Panjabi et al. [30] highlighted its importance based on an in vitro study where they showed that dynamic canal encroachment in a burst fracture during injury increased substantially by 85% compared with static canal encroachment at the time of imaging. Thus, evaluating the CER ratio on MRI would be optimal because it provides more information about the intervertebral disc and PLC in cases of LLFs. In this study, the optimal cutoff for CER was 50%, which is similar to previous reports.
In patients with substantial spinal canal stenosis, the CSAT is the most important MRI parameter that determines the severity of the canal stenosis. In LLFs, the CSAT must be measured because the spinal canal diameter provides a rough estimate of the space available for the nerve rootlets. In addition, the presence of the epidural hematoma with buckling of the ligamentum flavum significantly reduces the CSAT, causing neurological weakness. In this study, the optimal value of CSAT for determining neurological deficits was 65.3 mm2. Moreover, 33 (64.7%) of 51 patients with neurological deficits had an epidural hematoma. In these patients, the presence of epidural hematoma significantly reduced CSAT, which necessitated emergency decompression.
This study has a few limitations. First, this is a retrospective study with a relatively smaller sample size; thus, bias is possible. To validate the results of this study, a multicentric prospective study with a relatively larger sample size is necessary. Second, only patients who had undergone surgery were analyzed. Therefore, we may be unable to conduct an appropriate analysis because of insufficient data from the nonsurgically treated group. Third, given the potential for human errors due to manual measurements, an automated software tool may provide more reliable data. Finally, as the patients presented to our center at different periods, analyzing the factors that can predict neurological recovery was quite difficult.
The results of this study suggest that MRI parameters, namely, the CER and CSAT, are important radiological parameters for predicting the incidence of neurological deficits in patients with LLFs, with an optimal cutoff of 50% and 65.3 mm2. Moreover, the higher incidence of traumatic epidural hematomas in LLFs requires preoperative MRI in patients with neurological deficits to plan the appropriate extent of decompression.
Fig. 1
Midsagittal computed tomography image showing (A) measurement of anterior vertebral height (AVH) and posterior vertebral height (PVH). Loss of vertebral body height (LOVBH) is calculated as anterior/posterior ratio. RFF is measured as the distance from the posterosuperior corner of the injured vertebral body to a tangent line touching the posterior inferior cortex of the cephalad vertebral body. (B) Local kyphosis (LK) measured as the angle formed between the lines drawn along the superior and inferior end plate of the fractured vertebra. RFF, retropulsion of fracture fragment.

Fig. 2
Axial computed tomography images showing (A) measurement of interpedicular distance (IPD), (B) measurement of canal compromise (CC), and (C) measurement of sagittal transverse ratio (STR). SD, standard deviation.

Fig. 3
T2 weighted (A) midsagittal magnetic resonance imaging images showing measurement of canal encroachment ratio (CER). CER was calculated using the anteroposterior canal diameter of the fractured vertebra (DI) and the vertebrae above (DA) and below (DB) using the formula:
C E R = 1 - [ DI ( DA + DB ) ] × 100 %
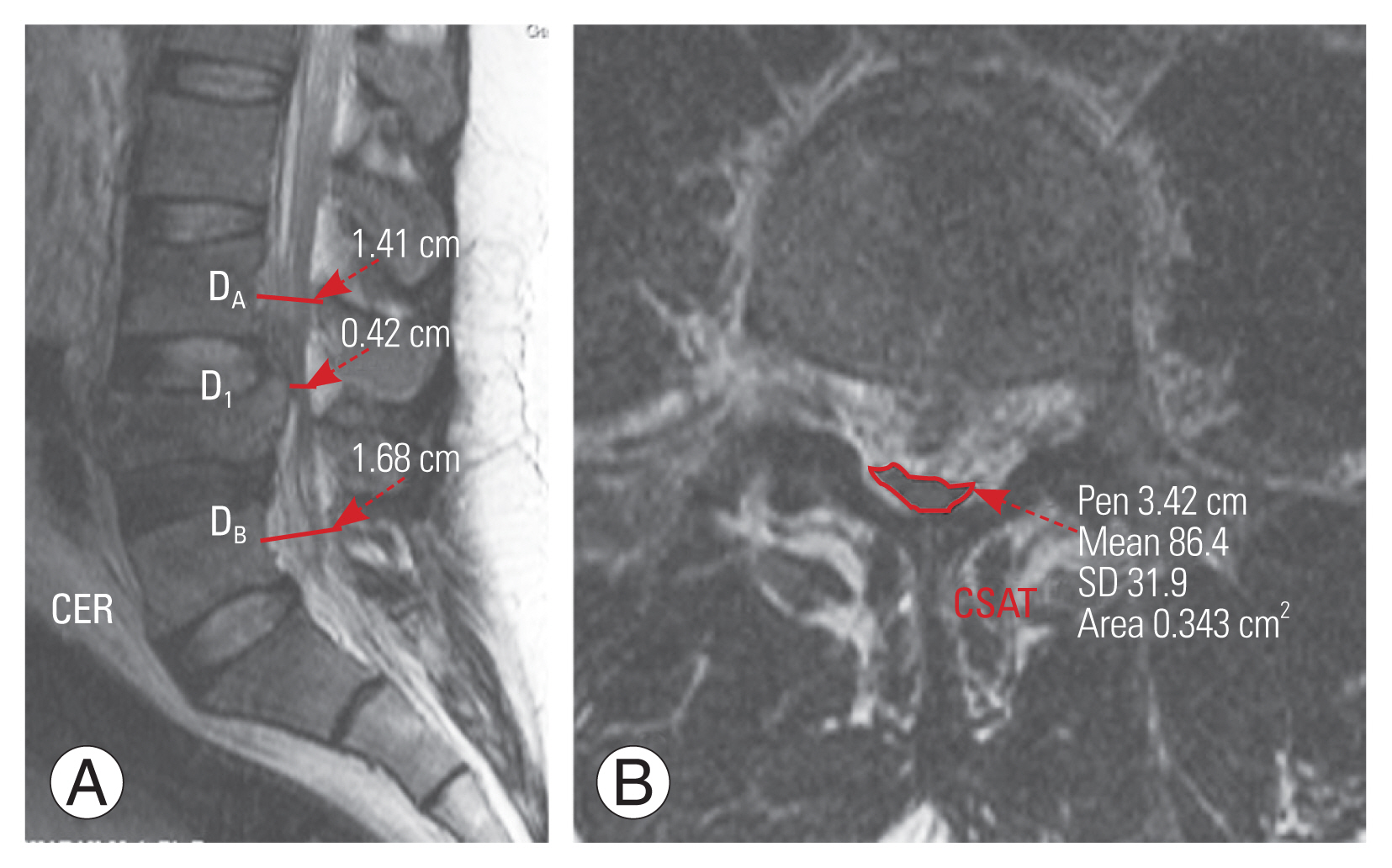
Fig. 4
T2 weighted magnetic resonance imaging. (A) Midsagittal and (B–D) axial images showing burst fracture at L3 level and extent of epidural hematoma up to L1 level.
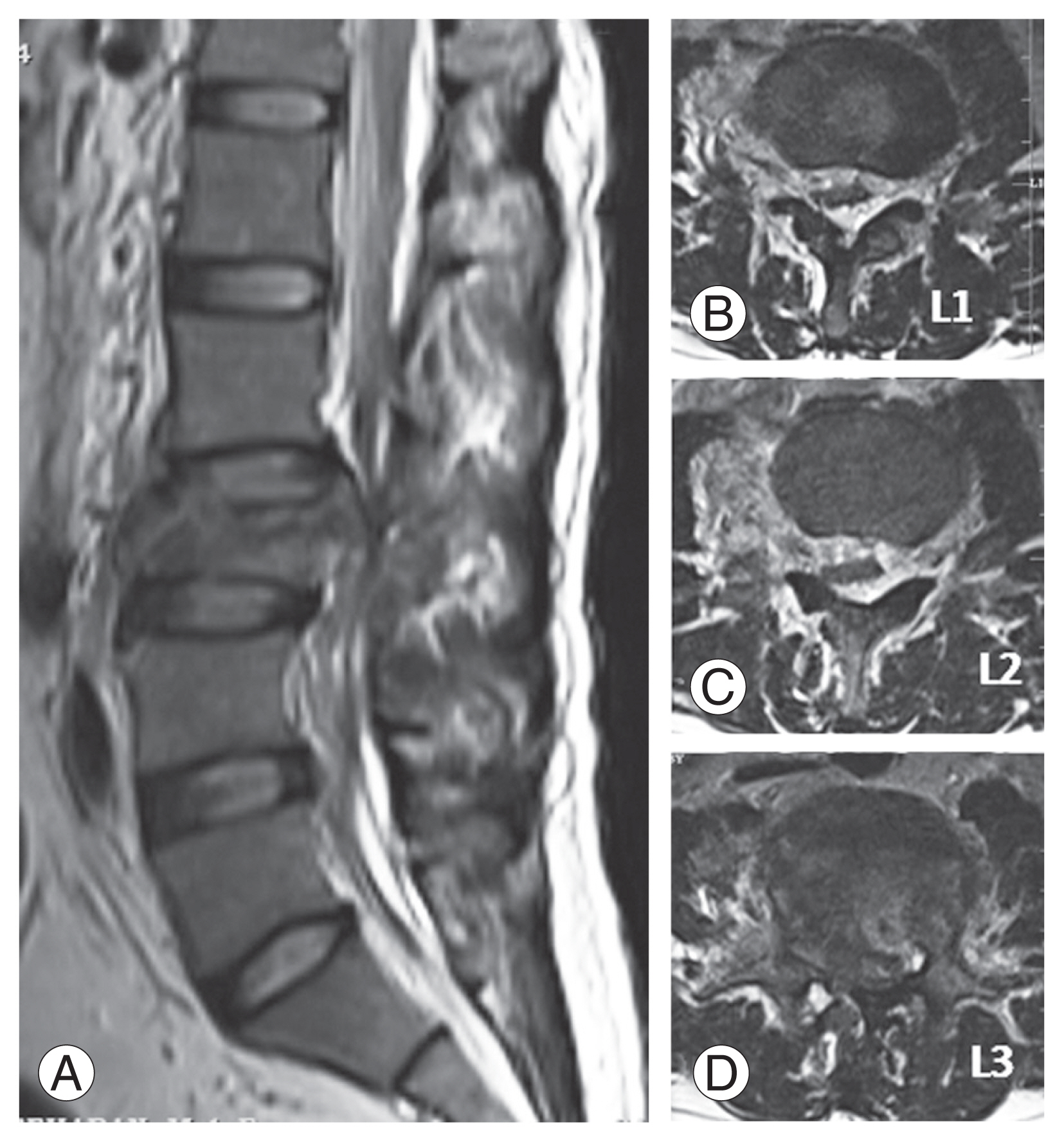
Table 1
Demographic and injury characteristics
Table 2
Radiological parameters determining the incidence of neurodeficit
Table 3
Univariate analysis
Table 4
Multivariate analysis
References
1. Seybold EA, Sweeney CA, Fredrickson BE, Warhold LG, Bernini PM. Functional outcome of low lumbar burst fractures: a multicenter review of operative and nonoperative treatment of L3–L5. Spine (Phila Pa 1976) 1999;24:2154–61.

2. Reinhold M, Knop C, Beisse R, et al. Operative treatment of 733 patients with acute thoracolumbar spinal injuries: comprehensive results from the second, prospective, Internet-based multicenter study of the Spine Study Group of the German Association of Trauma Surgery. Eur Spine J 2010;19:1657–76.




3. Moore TA, Bransford RJ, France JC, et al. Low lumbar fractures: does thoracolumbar injury classification and severity score work? Spine (Phila Pa 1976) 2014;39:E1021–5.

4. Sansur CA, Shaffrey CI. Diagnosis and management of low lumbar burst fractures. Semin Spine Surg 2010;22:33–7.

5. Rahimi-Movaghar V, Vaccaro AR, Mohammadi M. Efficacy of surgical decompression in regard to motor recovery in the setting of conus medullaris injury. J Spinal Cord Med 2006;29:32–8.



6. Werner BC, Yang S, Shen FH, Shimer AL. Cauda equina in the setting of thoracolumbar trauma: is early decompression indicated? Semin Spine Surg 2012;24:226–34.

7. Antiga S, Adajay KA, Anwar F, Vergara P. Against the odds: extraordinary recovery from complete cauda equina syndrome following L3 fracture: time still matters. Spinal Cord Ser Cases 2016;2:16027.




8. Harrop JS, Hunt GE Jr, Vaccaro AR. Conus medullaris and cauda equina syndrome as a result of traumatic injuries: management principles. Neurosurg Focus 2004;16:e4.

9. Talbott JF, Huie JR, Ferguson AR, Bresnahan JC, Beattie MS, Dhall SS. MR imaging for assessing injury severity and prognosis in acute traumatic spinal cord injury. Radiol Clin North Am 2019;57:319–39.


10. Li Y, Huang M, Xiang J, Lin Y, Wu Y, Wang X. Correlation of interpedicular distance with radiographic parameters, neurologic deficit, and posterior structures injury in thoracolumbar burst fractures. World Neurosurg 2018;118:e72–8.


11. Tang P, Long A, Shi T, Zhang L, Zhang L. Analysis of the independent risk factors of neurologic deficit after thoracolumbar burst fracture. J Orthop Surg Res 2016;11:128.




12. Xu JX, Zhou CW, Wang CG, et al. Risk factors for dural tears in thoracic and lumbar burst fractures associated with vertical laminar fractures. Spine (Phila Pa 1976) 2018;43:774–9.


13. Mohanty SP, Bhat NS, Abraham R, Ishwara Keerthi C. Neurological deficit and canal compromise in thoracolumbar and lumbar burst fractures. J Orthop Surg (Hong Kong) 2008;16:20–3.



14. Skeers P, Battistuzzo CR, Clark JM, Bernard S, Freeman BJ, Batchelor PE. Acute thoracolumbar spinal cord injury: relationship of cord compression to neurological outcome. J Bone Joint Surg Am 2018;100:305–15.

15. Meves R, Avanzi O. Correlation between neurological deficit and spinal canal compromise in 198 patients with thoracolumbar and lumbar fractures. Spine (Phila Pa 1976) 2005;30:787–91.


16. Lee HD, Jeon CH, Moon SW, Chung HW, Park KH, Chung NS. Radiological risk factors for neurological deficits after traumatic mid and low lumbar fractures. Spine (Phila Pa 1976) 2020;45:1513–23.


17. Willen J, Anderson J, Toomoka K, Singer K. The natural history of burst fractures at the thoracolumbar junction. J Spinal Disord 1990;3:39–46.


18. Ramon S, Dominguez R, Ramírez L, et al. Clinical and magnetic resonance imaging correlation in acute spinal cord injury. Spinal Cord 1997;35:664–73.



19. Furlan JC, Kailaya-Vasan A, Aarabi B, Fehlings MG. A novel approach to quantitatively assess posttraumatic cervical spinal canal compromise and spinal cord compression: a multicenter responsiveness study. Spine (Phila Pa 1976) 2011;36:784–93.

20. Ramachandran K, Shetty AP, Naik AS, Kanna RM, Rajasekaran S. Cervicothoracic translational injury: radiological analysis and risk factors of spinal cord injury. Indian Spine J 2023;6:132–40.

21. Hashimoto T, Kaneda K, Abumi K. Relationship between traumatic spinal canal stenosis and neurologic deficits in thoracolumbar burst fractures. Spine (Phila Pa 1976) 1988;13:1268–72.


22. Naduvanahalli Vivekanandaswamy A, Kannan M, Sharma V, et al. Prognostic utility of magnetic resonance imaging (MRI) in predicting neurological outcomes in patients with acute thoracolumbar spinal cord injury. Eur Spine J 2020;29:1227–35.



23. Lehman RA Jr, Paik H, Eckel TT, Helgeson MD, Cooper PB, Bellabarba C. Low lumbar burst fractures: a unique fracture mechanism sustained in our current overseas conflicts. Spine J 2012;12:784–90.


24. Motten T, Raghavendra V, Ali N. Functional outcome of management of lower lumbar (L-3 to L-5) burst fractures: a multicentre study of 34 cases. Int J Orthop 2017;3:482–7.

25. Yugue I, Aono K, Shiba K, et al. Analysis of the risk factors for severity of neurologic status in 216 patients with thoracolumbar and lumbar burst fractures. Spine (Phila Pa 1976) 2011;36:1563–9.


26. Radcliff K, Su BW, Kepler CK, et al. Correlation of posterior ligamentous complex injury and neurological injury to loss of vertebral body height, kyphosis, and canal compromise. Spine (Phila Pa 1976) 2012;37:1142–50.


27. Trafton PG, Boyd CA Jr. Computed tomography of thoracic and lumbar spine injuries. J Trauma 1984;24:506–15.


28. Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976) 1983;8:817–31.


- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 1,390 View
- 205 Download
- Related articles in ASJ
-
Undiagnosed Peripheral Nerve Disease in Patients with Failed Lumbar Disc Surgery2018 August;12(4)






