 |
 |
- Search
| Asian Spine J > Volume 18(2); 2024 > Article |
|
Abstract
Purpose
This study aimed to identify imaging risk factors for stenosis in extended neck positions undetectable in preoperative neutral magnetic resonance imaging (MRI) and improving decompression strategies for cervical spine disorders.
Overview of Literature
Cervical disorders are influenced by various dynamic factors, with spinal stenosis appearing during neck extension. Despite the diagnostic value of dynamic cervical MRI, standard practice often uses neutral-position MRI, potentially influencing surgical outcomes.
Methods
This study analyzed 143 patients who underwent decompression surgery between 2012 and 2014, who had symptomatic cervical disorders and MRI evidence of spinal cord or nerve compression but had no history of cervical spine surgery. Patient demographics, disease type, Japanese Orthopedic Association score, and follow-up periods were recorded. Spinal surgeons conducted radiological evaluations to determine stenosis levels using computed tomography myelography or MRI in neutral and extended positions. Measurements such as dural tube and spinal cord diameters, cervical alignment, range of motion, and various angles and distances were also analyzed. The residual space available for the spinal cord (SAC) was also calculated.
Results
During extension, new stenosis frequently appeared caudal to the stenosis site in a neutral position, particularly at C5/C6 and C6/C7. A low SAC was identified as a significant risk factor for the development of new stenosis in both the upper and lower adjacent disc levels. Each 1-mm decrease in SAC resulted in an 8.9- and 2.7-fold increased risk of new stenosis development in the upper and lower adjacent disc levels, respectively. A practical SAC cutoff of 1.0 mm was established as the threshold for new stenosis development.
Conclusions
The study identified SAC narrowing as the primary risk factor for new stenosis, with a clinically relevant cutoff of 1 mm. This study highlights the importance of local factors in stenosis development, advocating for further research to improve outcomes in patient with cervical spine disorders.
A key aspect of cervical disorders is the involvement of dynamic factors, which have been well documented in the literature [1–5]. A critical consideration in the management of cervical disorders is the development of spinal stenosis caused by the pincer mechanism in the extended cervical position [4]. To adequately address this issue, identifying and decompressing potential stenosis sites during evaluation are essential [6–8]. Assessing the cervical spine in the extended position can help identify these sites, enabling more effective decompression and reducing the risk of insufficient decompression [6–8].
Dynamic cervical magnetic resonance imaging (MRI) is an extremely useful diagnostic tool for assessing the involvement of dynamic factors in cervical disorders [6–8]. However, in general clinical practice, cervical MRI in the neutral position is common [9,10]. This approach may not fully capture the extent of spinal cord (SC) compression during neck extension, leading to suboptimal surgical planning and postoperative outcomes.
This study aimed to investigate imaging findings that may be risk factors for the development of stenosis in the extended position in cases requiring surgery for cervical disorders, in which preoperative MRI is not feasible in the neutral position. If these risk factors are identified, surgeons can better predict the sites of stenosis that may become problematic during neck extension, allowing for more targeted and effective decompression strategies.
This study was conducted in compliance with the principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the Institutional Review Board (IRB) of Tokyo Dental College Ichikawa General Hospital (IRB approval no., 254). Written informed consent was obtained from all patients.
At our academic institution, 143 patients underwent decompression surgery for cervical spine disorders between January 2012 and December 2014 and were followed up for >12 months. The inclusion criteria were as follows: symptomatic cervical disorders with at least one clinical sign of myelopathy or radiculopathy, evidence of cervical SC or nerve compression on MRI or cervical computed tomography (CT) myelogram, and no history of cervical spine surgery. Decompression alone was not performed in patients with a local kyphosis of >20°, spondylolisthesis of >3.5 mm, and occupying ratio of ossification of the posterior longitudinal ligament of >60%.
Patients’ demographic data, namely, age, sex, body height, body weight, disease type, Japanese Orthopedic Association score, and follow-up period, were retrospectively obtained.
Five spinal surgeons independently performed radiological evaluations using digital imaging and communications in medicine viewer (Synapse ver. 4.1.0; FUJIFILM Medical, Tokyo, Japan). The intervertebral disc level with the disappearance of the subarachnoid space in the cervical neutral and extended positions was used to determine the stenosis level. Myelography was performed in nearly all patients undergoing surgery, and the disappearance of the subarachnoid space was determined by preoperative CT myelogram in the cervical neutral and extended positions (Fig. 1). However, for patients who could not undergo myelography because of allergies, etc., preoperative MRI was performed in the neutral and extended positions on the same day.
Initially, the location of the stenosis in the neutral position was evaluated. The development of new stenosis in the extended position in the nonstenotic intervertebral segment adjacent to the intervertebral segment with stenosis in the neutral position was investigated (Fig. 2).
At the upper and lower-disc levels adjacent to the stenosis site in the neutral position, the anteroposterior (AP) diameter of the dural tube (AP of the dura) and the AP diameter of the SC (AP of the SC) were measured on a T2-weighted sagittal MRI in the neutral position. This measurement was performed at the site with the least residual space for the SC (space available for the SC [SAC]) and was performed in a line orthogonal to the SC. The SAC was calculated as the difference between the AP of the SC and the AP of the dura (Fig. 2).
Standing lateral-view plain radiographs were obtained with the neck in the neutral position, flexion, and extension preoperatively. Neutral-position lateral radiographs were obtained with the patient standing comfortably, with the head facing forward, and gazing in the horizontal direction. Cervical alignment was determined on preoperative neutral radiographs using a classification proposed by Chiba et al. [9] and analyzed as (1) lordosis, (2) straight, (3) sigmoid, and (4) kyphosis. In this study, the C2–C7 angle was measured as the Cobb angle between the C2 and C7 vertebral bodies. A positive value in the C2–C7 angle indicates lordosis. The range of motion was calculated as the difference between the C2–C7 angle during flexion and extension. The angles associated with the C2–C5 and C5–C7 angles were measured in the same way as those for C2–C7. The C2–C7 sagittal vertical axis was defined as the distance between the C2 plumb line and the superior posterior corner of the C7 vertebral body. The T1 slope was determined as the angle between the superior endplate of T1 and the horizontal line.
The intraobserver reliability tested by the interclass correlation coefficient (ICC) (1, 1) was 0.917 (0.859–0.952). The interobserver reliability tested by the ICC (2, 1) was 0.916 (0.833–0.959).
Statistical analysis was performed using IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA). All values were expressed as the mean±standard deviation, and p<0.05 was considered significant. All independent variables between the two groups were compared using the t-test for continuous variables and the Mann-Whitney U test for discrete variables. A chi-square test was performed to compare the development of new stenosis. Logistic regression analysis was used for risk factor analysis of new stenosis. Initially, univariate analysis was used to determine the significance of various parameters with p<0.10 in comparisons between the two groups. Factors with p<0.10 in the univariate analysis were then included in the multivariate analysis. Receiver operating characteristic (ROC) curve analysis was performed to investigate the threshold for new stenosis using parameters detected as risk factors in the multivariate logistic regression analysis. The Youden index was used to determine the cutoff value for each parameter.
The number of patients and the location of stenosis at various intervertebral segments in a neutral position were investigated (Table 1). Four-level stenosis between C3/C4 and C6/C7 was the most common pattern, affecting 32 patients (91% of all four-level cases). The next most frequent was a two-level stenosis between C4/C5 and C5/C6 (n=29, 64% of all two-level cases). C5/C6 was the most common among single-level cases (n=14, 70% of single-level cases). For three-level cases, stenosis from C3/C4 to C5/C6 was the most frequent (n=18, 53% of three-level cases).
In the extended position, new stenosis developed most frequently at the upper level in C4/C5–C5/C6 and C4/C5–C6/C7 patterns in the neutral position, affecting six patients each. For the C4/C5–C5/C6 two-level cases, new stenosis developed in the extended position in 21% of the patients, whereas 43% of the patients with C4/C5–C6/C7 three-level stenosis experienced new stenosis in the extended position.
The highest number of new stenosis at the lower level in the extended position was 10, accounting for 56% of patients with C3/C4–C5/C6 three-level stenosis in the neutral position. The second most common was the C4/C5–C5/C6 two-level pattern (n=9, 31%).
The incidence rate of new stenosis at the upper adjacent level in the extended position was the highest (28%) when C3/C4 was at the upper adjacent level. However, no significant difference was found when compared with other locations (Table 3, Fig. 3).
Similarly, the incidence of new stenosis at the lower level in the extended position was the highest (67%) when C5/C6 was at the lower adjacent level; however, no significant difference was noted when compared with other locations (p<0.10). When C6/C7 was at the lower adjacent level, the incidence of new stenosis was 39%, which was significantly higher. Conversely, the incidence when C7/T1 was at the lower adjacent level was 6%, which was significantly lower.
The U (+) group developed new stenosis in the upper adjacent intervertebral disc level in the extended position, whereas the U (−) group did not. Statistically significant differences were observed in the AP of the dura and SAC. The U (+) group had lower AP of the dura and SAC (Table 4).
The L (+) group developed new stenosis in the extended position of the lower adjacent intervertebral disc level, whereas the L (−) group did not. Statistically significant differences were observed in the following parameters: level of the lower adjacent intervertebral disc segment, AP of the dura, AP of the SC, and SAC. The L (+) group had higher cephalad adjacent intervertebral disc levels. The L (+) group had lower AP of the dura, AP of the SC, and SAC (Table 4).
In the analysis of risk factors for new stenosis developing in the extended position, univariate analysis was performed on factors with a p-value of <0.10 in the U and L group analyses. Multivariate analysis was then conducted on factors with a p-value of <0.10 in the univariate analysis (Table 5).
In the univariate analysis for new stenosis in the upper adjacent intervertebral disc level, the AP of the dura and SAC were significant risk factors. In the multivariate analysis, only SAC was a significant risk factor. A 1-mm decrease in SAC resulted in an 8.9-fold increased risk of new stenosis development in the upper adjacent disc level (Table 5).
The adjacent disc level, AP of the dura, AP of the SC, and SAC were significant risk factors. In the multivariate analysis, AP of the dura and SAC were significant risk factors in the univariate analysis for new stenosis developing in the lower adjacent intervertebral disc level. A 1-mm decrease in the AP of the dura resulted in a 2.5-fold increased risk of new stenosis development in the lower adjacent disc level, whereas a 1-mm decrease in the SAC resulted in a 2.7-fold increased risk at the same location (Table 5).
The difference between the two measurements of the AP of the SC and AP of the dura by the same examiner was −0.07350±0.49112 mm. The 95% confidence interval for the difference in measurements was −0.30335 to 0.15635 mm.
Considering the ROC curves, the cutoff value of the SAC for new stenosis development in the upper adjacent intervertebral disc level was 0.8450 mm (area under the curve [AUC], 0.859; sensitivity, 0.992; specificity, 1.000). A SAC of 1.1250 mm had the sensitivity and specificity of 0.992 and 0.957, respectively. Clinically, a SAC of 1.0 mm is practical (Fig. 4).
The cutoff value of the SAC for new stenosis development in the lower adjacent intervertebral disc level was 0.9550 mm (AUC, 0.858; sensitivity, 1.000; specificity, 0.972). A SAC of 1.210 mm had the sensitivity and specificity of 1.000 and 0.944, respectively. A SAC of 1.0 mm appeared to be practical (Fig. 4).
Numerous studies have investigated dynamic compression factors in the cervical spine and have suggested that this dynamic compression contributes to the development of cervical spondylotic myelopathy [1–8]. This study aimed to predict new stenosis development in cervical extension based on the images of the cervical spine in a neutral position. While two studies have examined factors causing stenosis in flexion and extension based on images of the cervical spine in a neutral position [6,7], neither considered the SC status. Note that the SC size does not proportionally correspond to the spinal canal size, and the disproportion between the SC and the canal is thought to contribute to canal stenosis [10]. No previous reports have considered SC status as a factor in the development of new stenosis, which appears insufficient when evaluating factors in cervical canal stenosis. To our knowledge, this is the first to include SC status when considering the AP diameter of the SC.
A key finding from our study is that new stenosis in the cervical spine frequently appeared caudal to the site of stenosis in a neutral position. This finding is crucial because a study reported that new stenosis following decompression surgery for cervical spondylotic myelopathy often occurs caudally [11]. Our study results also demonstrated that the cervical spine is more susceptible to dynamic compression on the caudal side. C5/C6 and C6/C7 were identified as the most common sites of new stenosis, possibly related to the fact that C5/C6 and C6/C7 are frequently involved in the stenosis sites of cervical spondylotic myelopathy [12]. Similarly, adjacent segment diseases are more likely to occur when C5/C6 and C6/C7 are adjacent intervertebral levels following anterior cervical fusion surgery [13], suggesting that this area is inherently prone to dynamic compression and stenosis.
In the cervical spine, extension movement is pronounced in the lower cervical segments, such as C5–C7 [14]. This increased motion can lead to the development of new stenosis when the cervical spine is in an extended position. The greater mobility of the lower cervical vertebrae may make this region more prone to developing spinal stenosis, which can cause pain and neurological symptoms.
New stenosis development in the extended cervical position was not associated with the number of stenotic segments in the neutral position or with any of the alignment parameters examined. Multivariate logistic regression analysis revealed that the significant factor in new stenosis was the narrowing of the SAC. Previous reports have identified narrowing of the AP diameter of the dural canal, bulging disc, disc mobility in flexion and extension, and local kyphosis as risk factors for new stenosis development [6–8]. When taken together, our results suggest that the development of new stenosis is less related to factors of the cervical spine as a whole and emerges primarily from local factors.
Although the narrowing of the SAC remained as a significant risk factor for new stenosis in this study, setting a clinically straightforward cutoff value at 1 mm appears reasonable. This is because a 1-mm difference in the SAC can be considered significant in this study when taking into account the error in MRI measurements, and the sensitivity and specificity in the ROC curve at cutoff values of approximately 1 mm were nearly the same.
Because a site with only 1 mm of SAC is prone to be stenotic in extension, treating it as a stenosis site is safer. To avoid insufficient postoperative decompression, decompression should be considered for stenotic levels and narrowing canal levels with only 1 mm of SAC on neutral MRI.
This study has several limitations. First, the exclusive focus on Japanese subjects may limit generalizability. Second, the small sample size potentially reduces rigor and statistical power. Third, given the retrospective design, we could not provide data on the SC in the flexed position, which is a serious limitation. Assessing the cervical spine in flexed positions is essential to provide additional insights into its biomechanics and pathophysiology. The omission of intervertebral disc angles and slippage evaluation may affect the understanding of cervical spine characteristics. Thus, future studies should include imaging in larger, more diverse cohorts, including complete cervical spine dynamics, in the flexed position, and comprehensive local measurements.
This study identifies the narrowing of the SAC as the primary risk factor for developing new stenosis in extension, with a clinically relevant cutoff value established at 1 mm. It underscores the significance of local factors in developing stenosis and advocates for further research to enhance patient outcomes in managing cervical spine disorders.
Notes
Author Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by all authors. Ryoma Aoyama wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Fig. 1
Determination of the stenosis level. The stenosis level was defined as the level where the subarachnoid space disappeared in the sagittal or axial images of computed tomography myelography in the neutral and extended positions. (A–C) In this case, as shown in the left image, stenosis was seen at C4/5 in the neutral position, but no stenosis appeared at C5/6 (arrows). (D–F) In the extended position in the middle image, stenosis was seen at two intervertebral levels, C4/5 and C5/6 (arrows). (G) In this case, as shown in the magnetic resonance imaging image on the right, the stenosis was decompressed posteriorly.
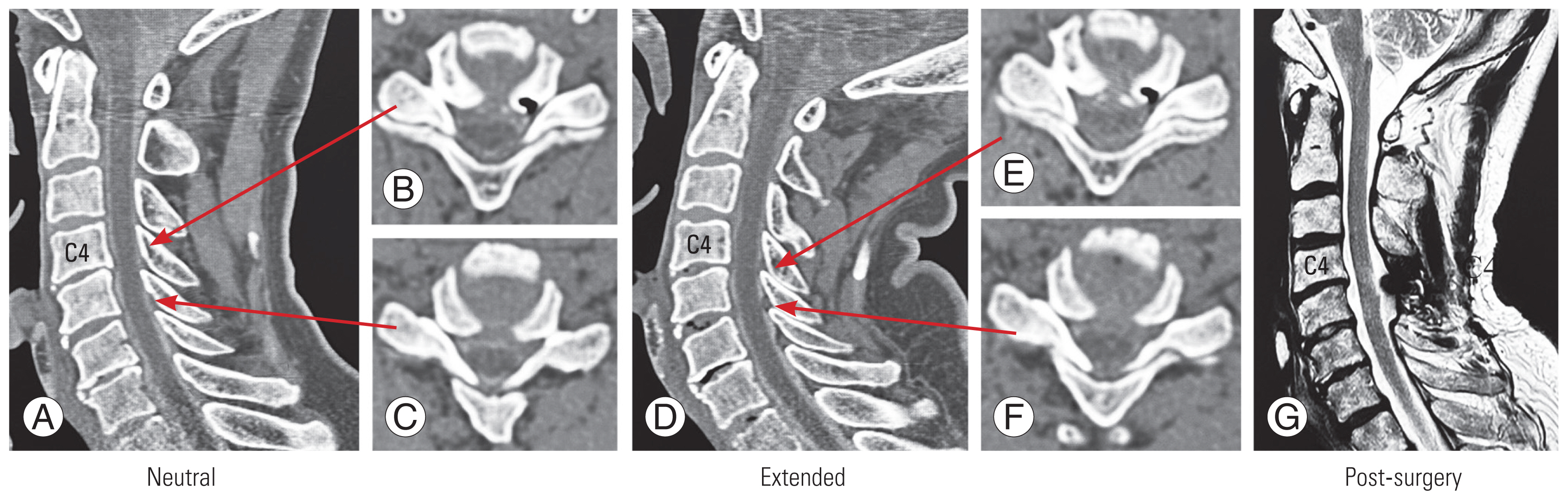
Fig. 2
Definition of the adjacent segment and measurement of the anteroposterior diameter. (A) The left picture shows the magnetic resonance imaging of a patient with two-level stenosis at C5/6 and C6/7 (arrowhead). Here, the upper adjacent segment is C4/5 (dotted line), and the lower adjacent segment is C7/Th1 (solid line). (B) The right picture shows the measurement of the anteroposterior diameter of the spinal cord (dotted line) and the dural tube (solid line) at the adjacent segment. The measurements were taken orthogonal to the spinal cord and at the site with the least residual space for the spinal cord at the disc level. Residual space available for the spinal cord (SAC) was calculated as the difference between the anteroposterior diameters of the spinal cord and the dural tube.
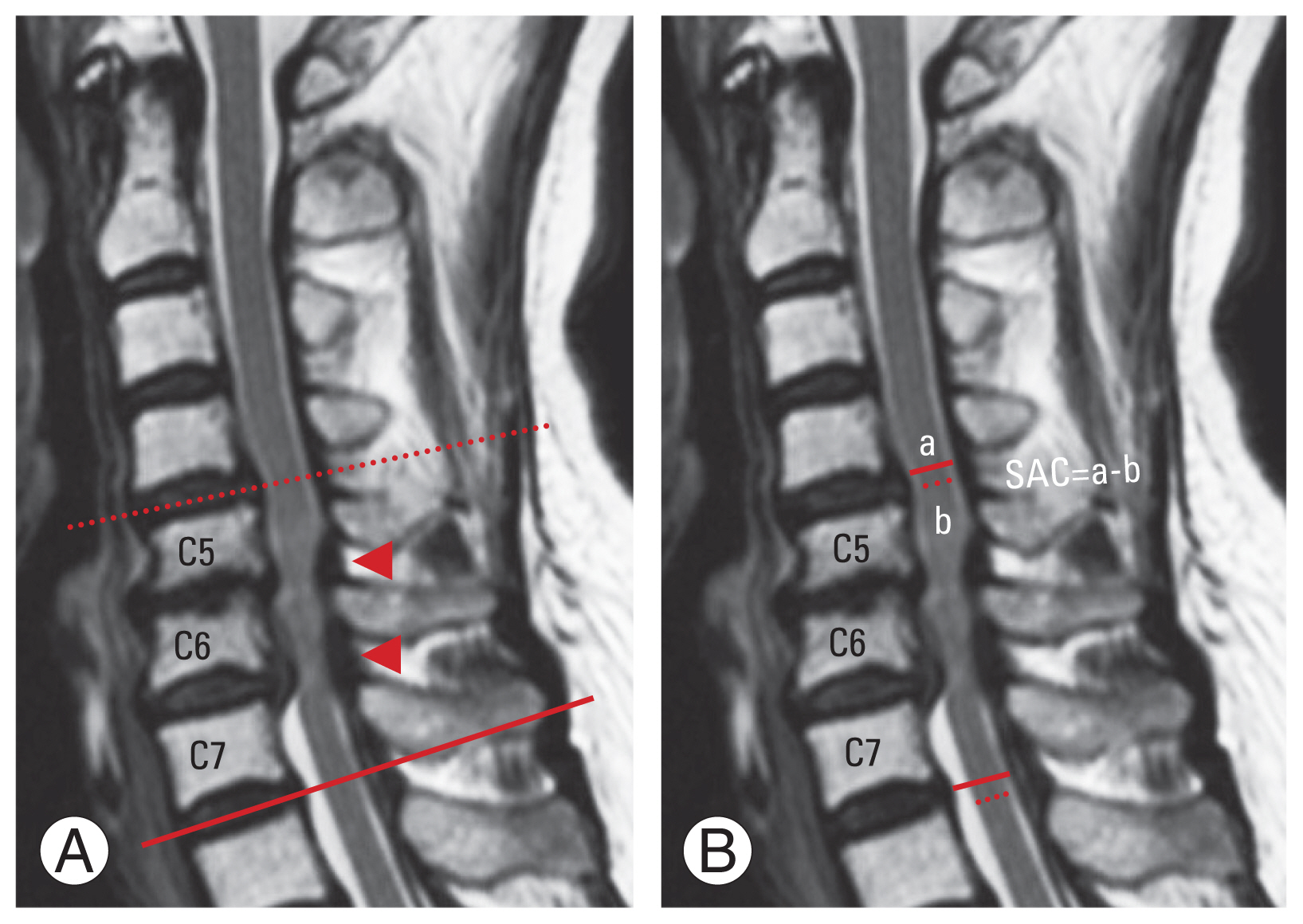
Fig. 3
Comparison of the incidence of new stenosis in an extended position. (A) As shown in the figure on the left, there was no significant difference in the incidence of new stenosis at adjacent levels in the extended position when compared by the number of stenosis levels in the neutral position. (B) When comparing the incidence of new stenosis in the extended position for each adjacent level, the incidence was higher at C3/4 in the upper adjacent level as shown in the middle figure, but the difference was not statistically significant. (C) The incidence at the lower adjacent level was higher at C5/6 as shown in the figure on the right. Statistically, the incidence was significantly higher at C6/7 and lower at C7/Th1. NS, not significant. *p<0.10. **p<0.05. ***p<0.01.
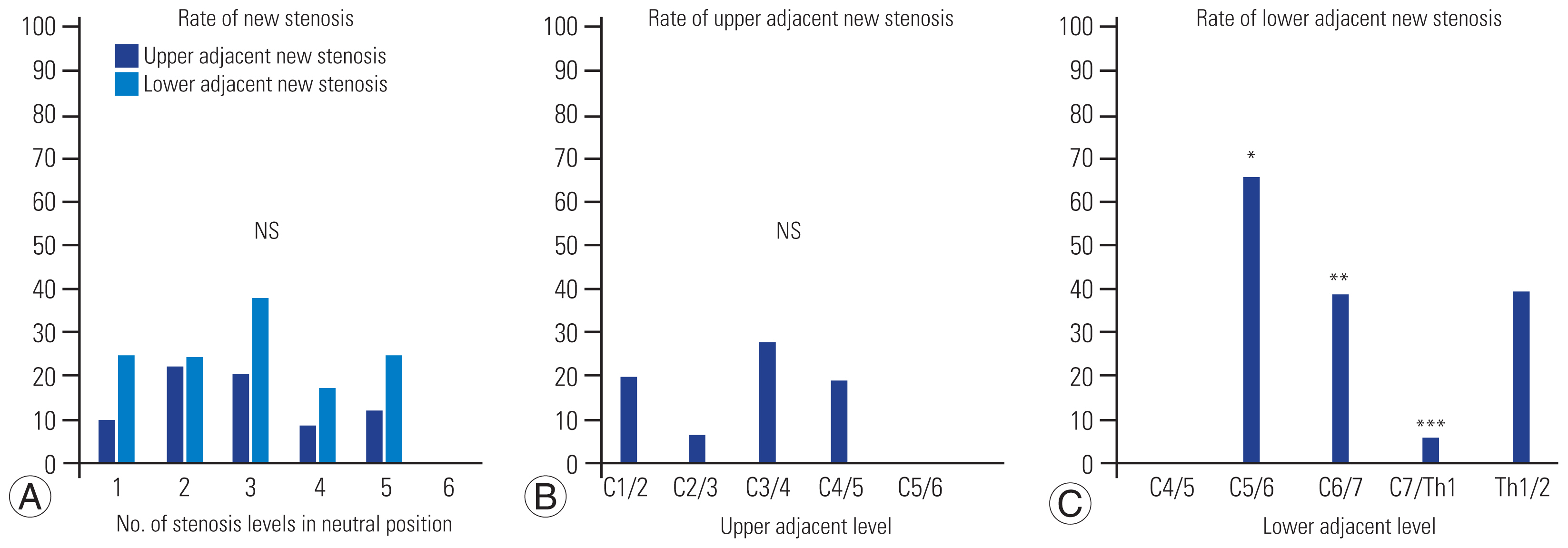
Fig. 4
Receiver operating characteristic (ROC) curve for upper and lower adjacent intervertebral segments to investigate a threshold for new stenosis using space available for the spinal cord. (A) On the left is the ROC curve for the upper adjacent intervertebral segment; area under the curve (AUC) was 0.859. (B) On the right is the ROC curve for the lower adjacent intervertebral segment; AUC was 0.858.
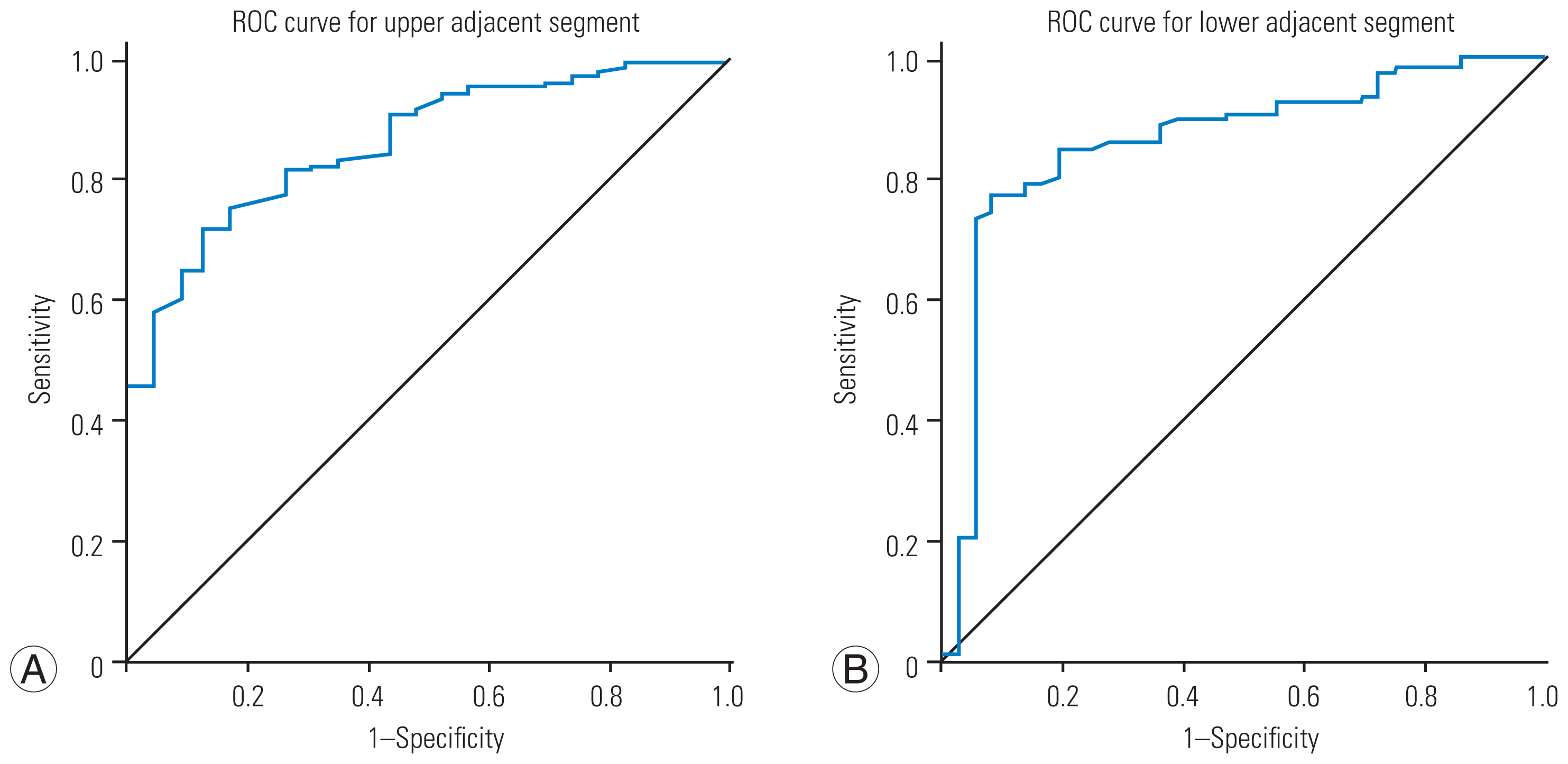
Table 1
A number of patients divided by the level of stenosis in the neutral position and the number and frequency of new stenosis at adjacent levels on the upper and lower side in the extended position
Table 2
The number of patients and frequency of new stenosis at adjacent levels in the extended position are divided by the number of stenosis levels in the neutral position
Table 3
A number of patients divided by the level of upper and lower adjacent levels and the number and frequency of new stenosis at the adjacent levels
Table 4
Comparison of parameters divided by the presence (+) or absence (−) of new stenosis at upper and lower adjacent levels in the extended position
| Variable | Upper adjacent new stenosis | Lower adjacent new stenosis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| U (+) | U (−) | p-value | L (+) | L (−) | p-value | |
| No. of patients | 23 | 120 | 37 | 106 | ||
|
|
||||||
| Adjacent disc level | 3.0±0.8 | 2.6±0.9 | 0.083* | 6.1±0.7 | 6.6±0.6 | 0.000 |
|
|
||||||
| No. of stenosis level | 2.6±1.0 | 2.8±1.2 | 0.437 | 2.7±1.1 | 2.8±1.2 | 0.628 |
|
|
||||||
| Age (yr) | 61.6±11.4 | 61.0±13.5 | 0.826 | 63.0±12.0 | 60.4±13.5 | 0.311 |
|
|
||||||
| Sex | 0.128 | 0.959 | ||||
|
|
||||||
| Male | 13 | 88 | 26 | 74 | ||
|
|
||||||
| Female | 10 | 34 | 11 | 32 | ||
|
|
||||||
| Body height (cm) | 162.0±8.1 | 163.4±9.1 | 0.482 | 163.4±8.3 | 163.1±9.1 | 0.857 |
|
|
||||||
| Body weight (kg) | 63.5±12.6 | 63.4±14.0 | 0.962 | 62.7±11.1 | 63.7±14.6 | 0.679 |
|
|
||||||
| Diseasea) | 17/3/0/2/1 | 81/23/5/5/6 | 0.878 | 26/6/3/1/1 | 72/20/2/6/6 | 0.374 |
|
|
||||||
| JOA score | 11.7±3.0 | 12.0±2.3 | 0.539 | 11.8±2.4 | 12.0±2.4 | 0.743 |
|
|
||||||
| AP of dural tube (mm) | 8.3±0.9 | 9.3±1.3 | 0.001 | 7.6±1.1 | 9.2±1.2 | 0.000 |
|
|
||||||
| AP of spinal cord (mm) | 6.2±1.0 | 6.2±0.9 | 0.939 | 5.4±0.8 | 5.8±0.7 | 0.024 |
|
|
||||||
| Space available for spinal cord (mm) | 2.1±0.5 | 3.1±0.9 | 0.000 | 2.2±0.8 | 3.4±1.0 | 0.000 |
|
|
||||||
| Alignmentb) | 2.3±1.2 | 2.2±1.2 | 0.660 | 1.9±1.1 | 2.3±1.2 | 0.095* |
|
|
||||||
| C2–7 angle (°) | 10.5±9.5 | 10.9±13.0 | 0.897 | 11.8±12.7 | 10.5±12.4 | 0.581 |
|
|
||||||
| Flexion C2–7 angle (°) | −10.8±6.5 | −10.6±11.6 | 0.950 | −8.0±10.4 | −11.6±11.0 | 0.084* |
|
|
||||||
| Extension C2–7 angle (°) | 25.2±9.7 | 24.1±12.9 | 0.697 | 25.0±13.6 | 24.0±12.1 | 0.962 |
|
|
||||||
| Range of motion (°) | 14.7±10.2 | 13.2±8.8 | 0.472 | 13.2±9.6 | 13.6±8.8 | 0.836 |
|
|
||||||
| C2–5 angle (°) | 3.2±9.7 | 4.0±10.6 | 0.721 | 5.4±9.5 | 3.4±10.7 | 0.297 |
|
|
||||||
| C5–7 angle (°) | 4.6±3.5 | 4.2±6.1 | 0.783 | 3.6±5.3 | 4.5±5.9 | 0.401 |
|
|
||||||
| C2–7 sagittal vertical axis (mm) | 20.6±13.5 | 20.4±12.4 | 0.953 | 20.4±11.4 | 20.5±13.0 | 0.978 |
|
|
||||||
| T1 slope (°) | 27.3±9.3 | 24.5±7.2 | 0.371 | 25.8±7.3 | 24.4±7.6 | 0.527 |
Table 5
Risk factor analysis of new stenosis development in extension
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Upper adjacent new stenosis | ||||
|
|
||||
| Adjacent disc level | 0.082* | 0.114 | ||
|
|
||||
| AP of dural tube | 0.499 (0.326–0.765) | 0.001 | 0.473 | |
|
|
||||
| SAC | 0.112 (0.042–0.300) | 0.000 | 0.112 (0.042–0.300) | 0.000 |
|
|
||||
| Lower adjacent new stenosis | ||||
|
|
||||
| Adjacent disc level | 0.350 (0.190–0.647) | 0.001 | 0.271 | |
|
|
||||
| AP of dural tube | 0.271 (0.164–0.450) | 0.000 | 0.404 (0.205–0.796) | 0.009 |
|
|
||||
| AP of SC | 0.544 (0.317–0.933) | 0.027 | 0.786 | |
|
|
||||
| SAC | 0.178 (0.090–0.354) | 0.000 | 0.372 (0.157–0.878) | 0.024 |
|
|
||||
| Alignment | 0.094* | 0.586 | ||
|
|
||||
| Flexion C2–7 angle | 0.083* | 0.427 | ||
References
1. Breig A, Turnbull I, Hassler O. Effects of mechanical stresses on the spinal cord in cervical spondylosis: a study on fresh cadaver material. J Neurosurg 1966;25:45–56.

2. Epstein NE, Hyman RA, Epstein JA, Rosenthal AD. “Dynamic” MRI scanning of the cervical spine. Spine (Phila Pa 1976) 1988;13:937–8.

3. Fukui K, Kataoka O, Sho T, Sumi M. Pathomechanism, pathogenesis, and results of treatment in cervical spondylotic myelopathy caused by dynamic canal stenosis. Spine (Phila Pa 1976) 1990;15:1148–52.


4. Penning L. Some aspects of plain radiography of the cervical spine in chronic myelopathy. Neurology 1962;12:513–9.


5. Taylor AR. The mechanism of injury to the spinal cord in the neck without damage to vertebral column. J Bone Joint Surg Br 1951;33-B:543–7.

6. Chen CJ, Hsu HL, Niu CC, et al. Cervical degenerative disease at flexion-extension MR imaging: prediction criteria. Radiology 2003;227:136–42.


7. Hayashi T, Wang JC, Suzuki A, et al. Risk factors for missed dynamic canal stenosis in the cervical spine. Spine (Phila Pa 1976) 2014;39:812–9.


8. Zhang L, Zeitoun D, Rangel A, Lazennec JY, Catonne Y, Pascal-Moussellard H. Preoperative evaluation of the cervical spondylotic myelopathy with flexion-extension magnetic resonance imaging: about a prospective study of fifty patients. Spine (Phila Pa 1976) 2011;36:E1134–9.

9. Chiba K, Toyama Y, Watanabe M, Maruiwa H, Matsumoto M, Hirabayashi K. Impact of longitudinal distance of the cervical spine on the results of expansive open-door laminoplasty. Spine (Phila Pa 1976) 2000;25:2893–8.


10. Ishikawa M, Matsumoto M, Fujimura Y, Chiba K, Toyama Y. Changes of cervical spinal cord and cervical spinal canal with age in asymptomatic subjects. Spinal Cord 2003;41:159–63.



11. Aoyama R, Shiraishi T, Yamane J, et al. Adjacent segment stenosis after muscle-preserving selective laminectomy: a retrospective study of patients with a minimum 10-year follow-up. Spine Surg Relat Res 2021;6:115–22.



12. Tani T, Ushida T, Taniguchi S, Kimura J. Age related shift in the primary sites of involvement in cervical spondylotic myelopathy from lower to upper levels. J Neurol Neurosurg Psychiatry 2002;73:316–8.










