Introduction
Non-dysraphic spinal intramedullary lipomas are extremely rare, constituting about <1% of all intraspinal tumors [1,2]. Lipomas of the spinal cord are considered to behave as spinal cord tumors that cause a neurological deficit as a result of their mass effect [3]. The current literature describing spinal cord lipomas frequently combines the adult and pediatric populations [4,5] and also includes intramedullary lipomas associated with dysraphism [6,7,8]. Non-dysraphic intramedullary spinal cord lipomas in adults are very rare [9]. Only eight cases of dorso-lumbar spinal lipomas without spinal dysraphism in adults have been reported in the English literature till 2013 [2,5,10]. Here we report our experience with three more cases of non-dysraphic adult spinal cord intramedullary lipomas in the dorsolumbar region and discuss the long term outcome compared with that of previously reported cases in the literature.
Materials and Methods
The medical records of 3 patients with non-dysraphic intramedullary lipomas of the spinal cord that were treated in our hospital over a period of 5 years (2006-2010) were retrospectively analyzed. All the three patients were male patients and the mean age at presentation was 25.3 years (range, 25-26 years). These patients presented with a neurological deficit. They were symptomatic for more than two years before the diagnosis. They presented with low backache and dysesthesia with a progressive neurological deficit. None of these patients had external neurocutaneous markers of spinal dysraphism (Table 1).
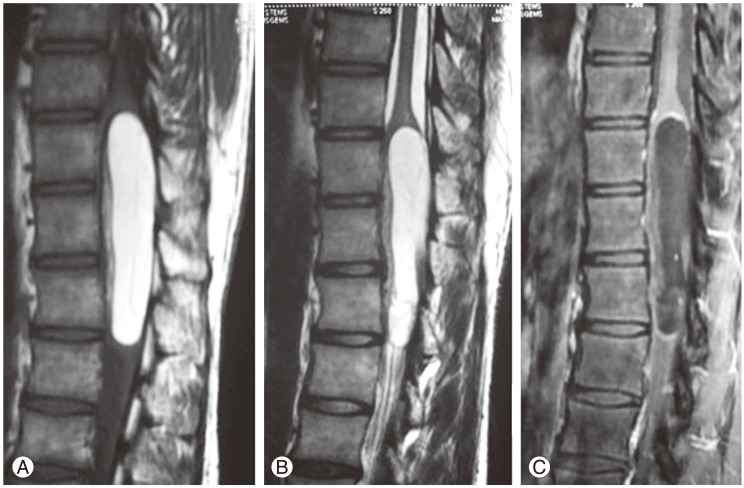
These patients were evaluated using magnetic resonance imaging (MRI) of the whole spine, which was performed on a 1.5-tesla machine (GE Healthcare, Chennai, India) with a conventional spin-echo protocol in the sagittal, coronal, and axial planes. The images were obtained in T1-weighted and T2-weighted sequences and in T1-weighted fat-suppression sequences. Computerized tomography of the involved spinal region was performed to rule out a congenital vertebral anomaly. A preoperative diagnosis of intramedullary lipomas was made based upon the MRI features. These were a uniform hyperintense signal on T1-weighted and T2-weighted images and a hypointense signal suggestive of a lipoma in the fat suppression sequences (Figs. 1, 2). The lipomas were located in the lumbar, dorsolumbar and dorsal regions, each one spanning two to three vertebral segments. The involved spinal cord was displaced anteriorly. The third case had been operated on 6 years ago for the same problem and the patient had transient improvement in his neurological status. On follow-up over the years, he had recurrent tumor, and because of this, his motor power gradually deteriorated, urinary incontinence developed, and he became paraplegic, with grade 2/5 motor power on admission (Table 1).
1. Surgical technique
All the cases were operated on by a senior author, with the patient under general anesthesia and lying in the prone position. The appropriate level was localized with an image intensifier, and the midline was exposed, and a laminectomy that extended one level above and one level below the level of the intramedullary tumor was performed. Before opening the dura mater, an injection of methylprednisolone at a dose of 30 mg/kg body weight was given intravenously as a bolus infusion. Under an operating microscope, the dura mater was opened in the conventional manner from 1 cm below the suspected lower margin of the tumor to 1 cm above the upper margin of the tumor. Dural traction sutures were applied using 4'0 vicryl on either side at regular intervals. The arachnoid mater which was observed as a pale white stretched sheet was opened with an arachnoid knife.
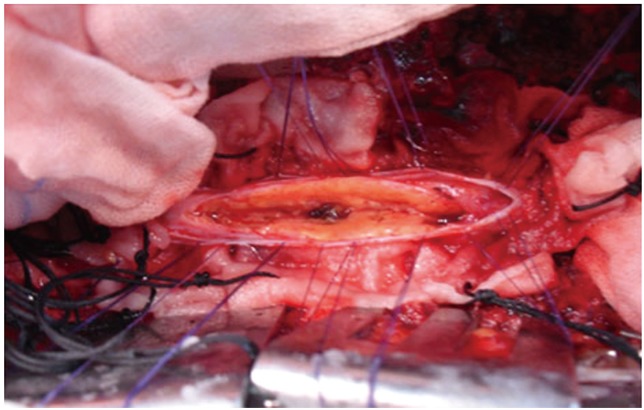
The cord was expanded in all cases and it was pale yellowish-white in color. Midline myelotomy was performed starting from the center of the maximum area of the bulge of the spinal cord or the yellowish discolored area and proceeded on either side with either a sharp 11 blade knife or a sharp arachnoid knife and pial sutures using 6'0 vicryl on either side. Only small vessels in the line of the myelotomy were sacrificed by coagulation. No large vessels in the posterior surface of the spinal cord were sacrificed throughout the surgery. A tumor was noted in all the 3 cases, 1-2 mm below the normal posterior surface of the spinal cord in the midline. Both the poles of the tumor were exposed.
The tumor was completely intramedullary in all the patients. The tumor was seen as yellowish (Fig. 3) and fluffy soft to firm in consistency. The lipoma-cord interface could not be established intraoperatively in any of the three patients (Fig. 3). Since a carbon dioxide laser was not available in our center, we used conventional microsurgical techniques. With meticulous surgical techniques, piecemeal intratumoral debulking was done using micro scissors, avoiding undue mechanical traction which could cause damage to the neural elements. In this context, we found curved micro scissors to be useful for excising the tumor piecemeal since they can be used parallel to the normal spinal cord without damaging the long tracts, especially the pyramidal tract. The other instrument we found extremely useful was the Sundt Flow Regulated Suction tube with variable tips (Scanlon) which could be used both as a suction tube and a dissector to gently dissect the fatty tissue from the cord. The pressure with which the tumor tissue was sucked could be controlled by partially occluding the trifoliate shaped lumen in the connector tube. A microtipped right angled 3 mm length ball tipped probe was another instrument which we found useful in dissecting the lipomas from the spinal cord, which was done with gentle dissection at the fat-cord junction.
We suggest not placing undue traction on the tumor tissue while removing it, especially when it is located in the deeper part of the anterior aspect of the cord. Similarly, we recommend not using fine cup shaped curettes which are normally used to do internal decompression in large extramedullary tumors of the spinal cord. This is because these maneuvers could cause traction over the anterior spinal artery complex leading to ischemic myelopathy and postoperative neurological deficits.
Minimal bleeding can be controlled by gentle normal saline irrigation and gentle compression with a small cottonoid kept over the bleeding point for few minutes. If bleeding persists, a small bit of oxidized cellulose (Surgicel, ETHICON, Johnson and Johnson, Somerville, NJ, USA) can be kept over the bleeding area and later removed with a saline wash. If profuse bleeding occurs, which is likely to occur at the junction of the apex of the tumor with the normal conus area, coagulation can be performed under low power after precisely identifying the bleeding spurter point. If needed, oxidized cellulose can be kept over the bleeder, and coagulation can be done over it. No attempt was made to define the tumor-cord interface. The tumor tissue was sent for histopathological examination. After radical subtotal excision of the tumor, the cord spontaneously collapsed in size.
Since intraoperative monitoring was not available in our institute, the spinal cord was observed for absence or decrease in cord pulsation during the entire surgical procedure. Whenever there was a mild decrease in the rate of cord pulsation or when it was doubtful, we stopped dissection for 10 to 15 minutes and then restarted it again. A gentle saline wash was given to clear any blood debris in the subarachnoid space. The end point of our tumor dissection was the observation of white matter substance on either side as this was likely to be the normal spinal cord tract; another end point was the complete collapse of the cord into the spinal canal to the extent where it would be easy to resuture the dura mater. There was always free flow of cerebrospinal fluid (CSF) until the end of the surgery. The arachnoid was left open and the dura mater could be easily closed in all cases after obtaining complete hemostasis. These maneuvers were the ones we used in all our cases, achieving radical subtotal excision of the intramedullary lipomas without producing new post-operative neurological deficits in any of the three cases.
2. Histopathological examination
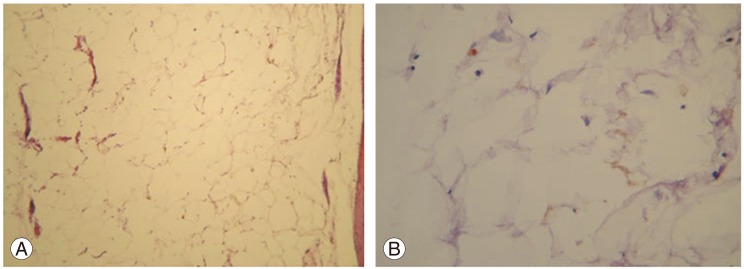
The tumor samples from these three patients were sent as multiple fragments of pale yellow soft tissue. They were fixed in 10% formalin, processed, and the 3 to 4 micron sections were stained with H&E stain. On light microscopic examination, all the three tumors were composed of lobules of mature adipose tissue and fibrous septae (Fig. 4).
No angiomatous or neuroglial elements were seen in any of the three tumors. Thus, the diagnosis of lipoma was confirmed in all of the patients.
Results
The postoperative period was uneventful in all of the three cases. Neurological improvement with respect to both motor and sensory systems was observed in two of the three cases. The third case had developed paraplegia with grade 2/5 motor power even before surgery, and he remained in the same state after surgery (Table 1). Among the two patients who had urinary incontinence prior to surgery, one with a motor power of 4/5 in the postoperative period attained continence of urine while the other with grade 2/5 motor power was still using a bladder catheter.
1. Follow-up
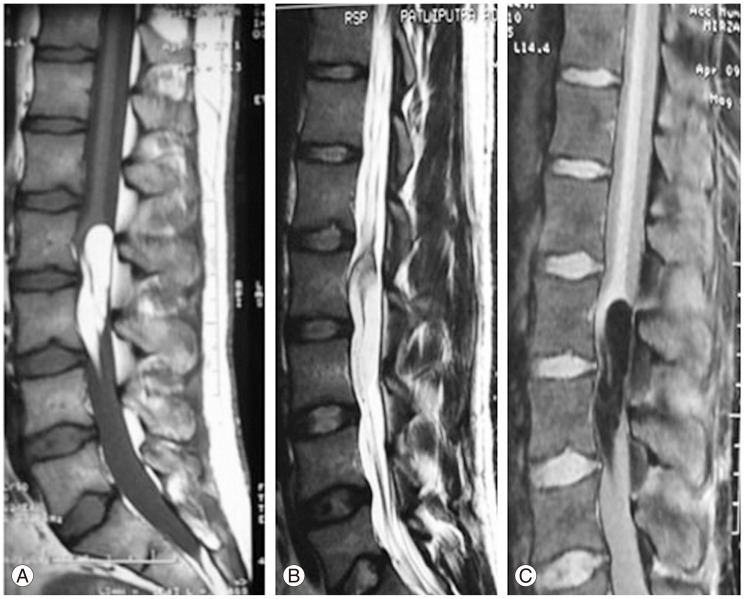
A postoperative MRI was performed in all of the three patients at the end of 4 weeks, and this showed significant reduction in the tumor size in all the three cases. These patients were followed up at regular intervals for a minimum period of 2 years. None of the patients worsened in the postoperative period.
Discussion
Spinal lipomas are usually associated with spinal dysraphism. Here, a subcutaneous lipoma communicates with the intraspinal component through a defect in the posterior neural elements. Non-dysraphic intramedullary spinal cord lipomas are very rare, especially in adults [1,2,5,10]. Most of the reported cases in the literature are located in the cervico-dorsal region and occur in children [2,4,5,11,12,13,14,15,16,17,18]. Perhaps spinal cord intramedullary lipomas are best considered as congenital inclusion tumors of the central nervous system. Several hypotheses are proposed for the origin of these tumours [4,16,17,18]. But Fleming et al. [18] believe that these lipomas occurring in non-dysraphic individuals represent true neoplasms because there is a progressive increase in tumor size over time due to hyperplasia, which was observed in two of their cases where recurrence occurred. They opine that lipomas that are present with dysraphic lipomatous malformations, that is, closed neural tube defects, are actually hamartomatous in nature [18].
Intramedullary lipomas have bimodal presentation. They may present during infancy and early childhood. They often present with a neurological deficit, which is thought to be due to birth trauma to the spinal cord [9,14]. More commonly they present in the third or fourth decade of life. All our patients were in this age group. Symptoms may be subtle, with slowly progressive myelopathy. Symptoms may worsen with proliferation of fat cells and deposition of more fat along the lesion [18]. These patients present with non-specific dysesthesias with dissociated sensory loss and long tract signs. Motor involvement occurs late in these cases.
MR imaging is the investigation of choice for spinal tumours. An intramedullary lipoma can be diagnosed with certainty in MRI scans since it is seen as a uniform hyperintense signal on T1-weighted and T2-weighted images and as a hypointense signal on fat suppression images (Figs. 1, 2). The level and extent of the tumour can be noted. Some believe that in almost every case, these lipomas are located over the posterior aspect of the cord, most frequently over the cervicodorsal region [5]. Multiple non-contiguous lipoma occurring all along the spinal cord has been reported [19,20]. In all of our three cases, the tumor was observed either in the dorsal region or the epiconus-conus region and they were operated on before a fixed neurological deficit occurred.
Surgical planning to note the location and extent of the tumour is important as the safety margin for neurological preservation is thin. Myelotomy is performed either in the midline or over the thinnest overlying neural tissue. Pial sutures are inserted for retraction and the lipoma is debulked. The end point of tumor resection is the stage when either the white matter of the spinal cord is seen in the lateral aspect on either side or the cord collapses completely to the extent where the dura mater could be closed without tension. Intraoperative ultrasonography may be helpful to define the cranial and caudal extent of the lesion and the definite plane of cord-tumour cleavage. A meticulous surgical technique is mandatory for neural preservation. Some authors have recommended the use of carbon dioxide laser as the laser vaporizes the fatty tissue without physical manipulation of the neural tissue [5,18].
Two of the largest case series reported in the literature in adults are by Bhatoe et al. [5] (14 cases) and Pruthi and Devi [10] (8 cases). In their case series, there was improvement in the neurological status in the majority of cases. There was no worsening of the motor deficit in their combined case series of 20 patients, excluding 2 children in the series of Bhatoe et al. [5]. Indeed there was improvement in motor power in four of 14 patients in Bhatoe et al.'s [5] series. In one of the Pruthi and Devi's [10] cases, even with partial decompression of the lipoma, it was noted that the neurological status could be preserved for a long duration, even up to 11 years [10]. There was improvement in the sensory symptoms including dysesthesia in the majority of cases (11/14 of Bhatoe et al. [5]). Similar improvement was noted in 2 of our 3 of our cases and also in various case reports reported earlier in the literature [2,14,20]. Lee et al. [4] in their study involving 6 patients, reported that these group of patients present with significant neurological compromise and show no improvement with surgical resection. We concur with this observation since in our third case there has been no significant improvement in motor power even on long term follow-up since he first presented with a motor power of 2/5 with bladder involvement. Variable outcomes have been reported with regards to the outcome after surgery [21]. A few reports have stated that only decompressive laminectomy [4,17] or decompressive laminectomy with biopsy only may be sufficient to stabilize the disease process, something that we do not agree with [22]. A detailed review of the literature states that surgical intervention after the onset of symptoms is unfavorable because of significant postoperative residual disability [4,5,10,18]. Motor improvement is seen in 40% of symptomatic patients after surgery, and bladder control is achieved in only a minority of these patients [7,16]. In one of our cases, even bladder disturbance improved on follow-up which is at variance with the above results. We opine that early surgical intervention with radical debulking of the intramedullary lipomas even before a fixed motor deficit occurs will lead to stabilization of the disease process and preservation of the motor function for a longer period. Correlating our third case with our other cases of spinal cord tumors on which we had operated and had followed up over several years, we wish to state that when the motor power is reduced to 2/5, the chances of recovery of both the motor power and bladder continence is unlikely.
Although resection of lipomas has been demonstrated to be effective treatment for these lesions [5,10,18,20,23], recurrence is an issue. Review of the literature shows only one case of re-surgery in an adult after 11 years [10]; this is because the postoperative follow up time was variable and recurrence was not reported often. In children, the recurrence has been reported by Lee et al. [4] (2 cases) and Fleming et al. [18] (2 cases among 5). In Fleming et al.'s [18] cases, the re-surgery performed stabilized the disease process with a recurrence-free interval of more than 3 years. Since these lipomas are very slow-growing tumors, the greatest drawback of MR imaging is its ineffectiveness postoperatively in precisely differentiating recurrence from residual tumor, which is always present in cases of intramedullary lipomas since total resection of these tumors is not feasible. Characteristically, the area in which the lipomas have been resected demonstrates a diffuse area of abnormality where the tissue planes are disrupted and the resulting scar tissue melds homogenously with the residual fibrofatty tissue of the lipomas [24]. In our third case, this patient underwent surgery 6 years ago and gradually deteriorated after initial improvement, but was reluctant to undergo repeat surgery. On follow-up, his motor power deteriorated from 3/5 to 2/5 and he became bedridden following which he consented to have re-surgery. But on follow-up after the second surgery, he did not show significant improvement in his motor power and the urinary incontinence remained. There was mild improvement in his sensory symptoms. Thus, these cases illustrate that in the case of clinical recurrence and not radiological recurrence, since residual tumor may mimic a recurrence, it is better to perform re-surgery and debulk the tumor at an early stage, even in adult cases, as this will further help preservation of neurological function for longer periods.
Conclusions
We believe that the long-term outcome after surgery is determined by the preoperative neurological status. Earlier surgical intervention with preserved neurological status results in a better outcome. Even with conventional microsurgical techniques as mentioned in the section under surgical technique, radical subtotal excision can be performed without producing a postoperative neurological deficit. Hence, we state that the long-term outcome for benign non-dysraphic intramedullary lipoma is good when the preoperative neurological status is preserved and meticulous conventional microsurgical techniques are used. When early clinical signs of recurrence develop, and these are supported by the radiological evidence, such patients should be re-operated on at the earliest opportunity to prevent the patient from developing a fixed neurological deficit in the long-term.