 |
 |
- Search
| Asian Spine J > Volume 17(6); 2023 > Article |
|
Abstract
Purpose
This study aims to determine whether preoperative neuroforaminal stenosis (FS) severity is associated with motor function patient-reported outcome measures (PROMs) following anterior cervical discectomy and fusion (ACDF).
Overview of Literature
Cervical FS can significantly contribute to patient symptoms. While magnetic resonance imaging (MRI) has been used to classify FS, there has been limited research into the impact of FS severity on patient outcomes.
Methods
Patients undergoing primary, elective 1ŌĆō3 level ACDF for radiculopathy at a single academic center between 2015 and 2021 were identified retrospectively. Cervical FS was evaluated using axial T2-weighted MRI images via a validated grading scale. The maximum degree of stenosis was used for multilevel disease. Motor symptoms were classified using encounters at their final preoperative and first postoperative visits, with examinations Ōēż3/5 indicating weakness. PROMs were obtained preoperatively and at 1-year follow-up. Bivariate analysis was used to compare outcomes based on stenosis severity, followed by multivariable analysis.
Results
This study included 354 patients, 157 with moderate stenosis and 197 with severe stenosis. Overall, 58 patients (16.4%) presented with upper extremity weakness Ōēż3/5. A similar number of patients in both groups presented with baseline motor weakness (13.5% vs. 16.55, p=0.431). Postoperatively, 97.1% and 87.0% of patients with severe and moderate FS, respectively, experienced full motor recovery (p=0.134). At 1-year, patients with severe neuroforaminal stenosis presented with significantly worse 12-item Short Form Survey Physical Component Score (PCS-12) (33.3 vs. 37.3, p=0.049) but demonstrated a greater magnitude of improvement (╬öPCS-12: 5.43 vs. 0.87, p=0.048). Worse stenosis was independently associated with greater ╬öPCS-12 at 1-year (╬▓=5.59, p=0.022).
Cervical neuroforaminal stenosis is often caused by underlying superior articular process hypertrophy, ligamentum flavum hypertrophy, or intervertebral disk herniation [1ŌĆō4]. While mild neuroforaminal stenosis is most likely asymptomatic, moderate-to-severe stenosis may cause radiculopathy and motor deficits. Management often depends on the severity and duration of the patientŌĆÖs clinical symptoms and their participation in nonoperative management. If the disease refuses to respond to conservative management, surgery is often required to improve the patientŌĆÖs quality-of-life [2]. Because decompression of the neural elements yields consistently excellent clinical outcomes, anterior cervical discectomy and fusion (ACDF) is the most commonly used operative treatment [5ŌĆō8].
Magnetic resonance imaging (MRI) is the modality of choice before surgery for determining the degree of neural element compression and the site of nerve impingement [9,10]. Although the clinical utility of MRI is widely established, literature on its capacity to predict clinical outcomes based on the degree of stenosis is limited. Kim et al. [9] previously attempted to classify the magnitude of nerve root compression on preoperative MRI; however, it is unknown whether the classification is prognostic for postoperative motor recovery and health-related quality-of-life outcomes.
While ACDF can promote neurologic and sensory recovery in up to 90% of patients [11], it is unclear whether improvements are preferentially observed in patients with severe neuroforaminal stenosis. One prior study on cervical stenosis found a correlation between neuroforaminal stenosis severity and the presence of neurologic manifestations [12], but the implications for how stenosis severity affects postoperative symptom resolution and patient-reported outcome measures (PROMs) are currently unknown. Therefore, the primary objective of our study was to determine whether the severity of preoperative neuroforaminal stenosis could be used to predict the recovery of motor function following ACDF. Our secondary objective was to determine if preoperative stenosis was associated with improved health-related quality-of-life metrics.
After approval from the Thomas Jefferson University Review Board (approval number: 19D.493), one of nine fellowship-trained spine surgeons conducted a retrospective cohort study on patients who underwent surgery at a single urban, tertiary referral academic medical center. The requirement for informed consent from individual patients was waived because of the retrospective nature of the study. Patients over the age of 18 years who underwent elective 1ŌĆō3 level ACDF for cervical radiculopathy between 2015 and 2021 were retrospectively identified and included in our analysis using Current Procedural Terminology (CPT) codes and a Structured Query Language search for CPT code 22551. Patients were excluded from the study if they lacked preoperative MRI images, were indicated for revision procedures, did not have a primary diagnosis of radiculopathy, or received surgical intervention for any malignant tumors, infections, or trauma.
Patient demographic data, including age, sex, body mass index (BMI), smoking status (never smoker, former smoker, or current smoker), Elixhauser Comorbidity Index, levels fused, and length of clinical follow-up, were collected using chart review. Motor symptoms were classified using patient clinic encounters at their final preoperative and first postoperative visits. PROMs were collected through the institutionŌĆÖs prospectively managed outcomes database (OBERD, Columbia, MO, USA). PROMs included the 12-item Short Form Survey Physical Component (PCS-12) and Mental Component (MCS-12) scores and the Visual Analog Scale (VAS) neck and arm pain scores.
A Smith-Robinson approach is used to gain access to the anterior part of the spine. After peanut dissection on the anterior longitudinal ligament, monopolar or bipolar cautery is used to elevate the longus colli muscles for self-retaining soft tissue retraction. Operative levels are radiographically confirmed. A discectomy is performed, and curettes and a high-speed burr are used to remove the cartilaginous endplates. Uncovertebral resection is performed with a high-speed burr and Kerrison rongeur if a far lateral disk is present. The posterior longitudinal ligament is removed along with any posterior osteophytes. A nerve hook is used to palpate the pedicle to determine the appropriate decompression width to ensure complete decompression.
Axial T2-weighted MRI images were used to evaluate cervical neuroforaminal stenosis at the symptomatic cervical level. Radiology reports of mild, moderate, or severe stenosis accompany typical MRI reports evaluating foraminal stenosis. While insurers use these to support an indication for surgical treatment, these reports are unreliable [10], as up to 10% of cases in symptomatic patients may be reported as normal [13]. Instead, the severity of stenosis was classified into one of three grades as described by Kim et al. [9], a validated neuroforaminal stenosis grading system. The scores were determined by evaluating the narrowest width of the neuroforamen compared with the width of the extraforaminal nerve root at the level of the anterior margin of the superior articular process of the affected vertebral body level. Grade 0 refers to the absence of stenosis with the narrowest width of the neural foramen greater than the width of the extraforaminal nerve root. Grade 1 refers to moderate cervical stenosis with the narrowest width of the neural foramen between 51% and 100% of the width of the extraforaminal nerve root. Grade 2 refers to severe cervical stenosis with the width of the neural foramen equal to or less than 50% of the width of the extraforaminal nerve roots (Fig. 1). Representative patient MRI images corresponding to grade 0ŌĆō2 neural foraminal stenosis are shown in Figs. 2ŌĆō4, respectively.
The level with the most severe neural foraminal stenosis was recorded as the highest stenosis severity in patients with multilevel cervical radiculopathy. This was performed for statistical analysis of PROMs because a PROM represents the entire patient experience rather than the effects of an individual level. However, neural foraminal compression was assessed on an individual level basis for myotomes corresponding to that cervical level for the assessment of motor weakness. For example, if a patient had grade 1 FS at C4ŌĆōC5 but grade 2 FS at C5ŌĆōC6, their PROMs were tabulated as grade 2 FS because that represented the maximal neural foraminal compression. However, weakness was assessed separately for C4ŌĆōC5 and C5ŌĆōC6 with respect to muscular action.
Patients were grouped according to the severity of their neuroforaminal stenosis. In the case of patients with multilevel radiculopathy, the maximum stenosis grade assigned to any level was used to tabulate the patient into a group. Descriptive statistics were used to represent patient demographics and outcome measures and were represented by mean┬▒standard deviation. Continuous variables were assessed using either an independent t-test or Mann-Whitney U test for parametric and nonparametric data. All categorical variables were compared using a Pearson chi-square analysis or FisherŌĆÖs exact test for small cell counts. Weakness was only considered on the same laterality as the MRI-defined stenosis. We defined weakness as any grade Ōēż3/5 consistent with prior literature [11,14]. Complete postoperative recovery was defined as postoperative strength of 5/5, whereas partial postoperative recovery was defined as a strength recovery that did not reach 5/5. For level analysis, motor weakness and recovery were considered only in relation to the affected foraminal stenosis level. A delta score (╬ö) was calculated for all PROMs, defined as the difference between postoperative and preoperative scores. Only PROMs for patients with absolute values were included for analysis. The delta score was used to compare postoperative outcomes to ensure that preoperative differences did not impact postoperative scores but represented the actual change in PROM values. Multivariate regression for ╬öPROMs was performed while controlling for patient age, sex, BMI, and the number of levels fused. All statistical analyses were performed using R Studio ver. 4.0.2 (RStudio, Boston, MA, USA). p-values <0.05 were considered statistically significant.
A total of 354 patients were included in the final cohort, with the following MRI grade groupings with 157 moderate (grade 1) stenosis and 197 severe (grade 2) stenosis patients. A total of 585 levels demonstrated FS, most commonly affecting C5ŌĆōC6 (N=247), followed by C6ŌĆōC7 (N=207), C4ŌĆōC5 (N=83), C3ŌĆōC4 (N=29), and C7ŌĆōT1 (N=19). No patients receiving radiculopathy surgery had any foraminal stenosis (grade 0). Patients with severe stenosis had higher rates of multilevel fusions (44.6% versus 69.5% single-level fusions, p<0.001) than patients with moderate stenosis. There were no significant differences between groups in terms of other patient or disease characteristics, such as symptom duration or the presence of concurrent myelopathy. Patients in both groups had similar hospital lengths of stay (1.92┬▒3.9 days versus 1.24┬▒0.4 days, p=0.107) and rates of home discharge (97.8% versus 99.0%, p=0.560) (Table 1). Overall, there were no significant differences between the groups in terms of complication rates (3.2% versus 0.5%, p=0.092), 90-day readmission rates (1.3% versus 2.0%, p=0.697), or revision surgeries (7.0% versus 10.2%, p=0.298).
Overall, 58 patients (16.4%) presented with upper extremity weakness Ōēż3/5. A similar number of patients in both groups presented with baseline motor weakness (13.5% versus 16.5%, p=0.431). Postoperatively, a similar number of patients experienced partial motor recovery. Although not statistically significant, more patients with severe stenosis experienced full motor recovery (97.1% versus 87.0%, p=0.134) (Table 2). When analyzing levels with foraminal stenosis individually, a similar number of affected levels were associated with motor deficits despite foraminal stenosis severity (12.9% versus 13.7%, p=0.781). Furthermore, both partial recovery and full motor recovery were similar in both groups (Table 3).
The overall PROM completion rate was 27.8% (N=97) for PCS-12 and MCS-12 and 20.1% (N=71) for VAS neck and VAS arm. Patients with severe neuroforaminal stenosis presented with significantly worse PCS-12 preoperatively (33.3±7.8 versus 37.3±9.0, p=0.049) but demonstrated a greater magnitude of improvement as measured by ΔPCS-12 scores (5.43±10.9 versus 0.87±11.3, p=0.048) at 1-year. Patients with severe stenosis also had higher VAS neck (3.47±2.9 versus 2.29±3.0, p=0.021) 1 year postoperatively, although a similar magnitude of improvement was similar between groups. There were no other differences in baseline, 1-year, or magnitude of improvement among health-related quality-of-life metrics (Table 4). The degree of neuroforaminal stenosis was an independent predictor for greater ΔPCS improvement at 1-year follow-up in multivariate linear regression (β=5.59, p=0.022) (Table 5).
Cervical radiculopathy affects 85 persons per 100,000 yearly [15], and ACDF remains the most commonly performed intervention for patients who fail conservative therapy, with nearly 130,000 performed annually in the United States alone [16]. While ACDF can provide excellent clinical outcomes for patients with cervical radiculopathy, the relationship between the severity of neuroforaminal stenosis and clinical outcomes is unknown. We evaluated clinical outcomes among patients undergoing ACDF for cervical radiculopathy and compared outcomes based on the severity of neuroforaminal stenosis. ACDF significantly improved motor function and PROMs for all patients. However, patients with more severe neuroforaminal stenosis present with poorer baseline physical functioning but exhibit greater clinically and statistically significant improvement in physical function. Literature evaluating outcomes based on assessments of cervical neuroforaminal stenosis is limited. Only one study, which evaluated the response to transforaminal steroid injections in patients with cervical radiculopathy, evaluated whether clinical improvement is impacted by the severity of the neuroforaminal stenosis [17]. The authors identified significant improvement regardless of the degree of stenosis, which is similar to our findings.
While patients with severe neuroforaminal stenosis were more likely to have reduced baseline physical function, ACDF improved physical functioning in all patients and promoted improvements in muscle strength regardless of the extent of nerve root compression. However, patients in our study with worse stenosis reported an even greater improvement in PCS-12, despite a similar degree of motor recovery. The reason for this discrepancy is unclear but is supported by other analyses of motor recovery and health-related quality-of-life after ACDF. A preliminary analysis found no relationship in preoperative, postoperative, or improvement in PCS-12 between patients with and without motor weakness undergoing ACDF [18]. Another analysis of patients undergoing ACDF for radiculopathy found that improvements in neither pinch strength nor grip strength correlated to postoperative improvements in PCS-12 [19]. This could be attributed to preoperative expectation counseling. Yee et al. [20] demonstrated that patients with greater preoperative expectations reported greater postoperative improvements in the 36-Item Short Form Health Survey physical component score following spine surgery. Patients with more severe stenosis presented with greater motor deficits, which may help explain the decrease in patient-reported physical function and the resultant increase at 1-year follow-up. Similarly, patients reported greater physical function (PCS) and motor recovery during the post-treatment clinical course in a preliminary analysis of motor weakness due to lumbar spine nerve impingement causing radiculopathy [21].
We found that physical motor improvement was unrelated to differences in arm or neck pain improvement, as patients had similar degrees of improvement in VAS arm and VAS neck regardless of the magnitude of neuroforaminal stenosis. VAS neck scores were worse at 1-year in patients with more severe foraminal stenosis. Interestingly, at baseline, patients had similar levels of pain, physical function impairment, and disability. These findings contradict a previous study that found stenosis severity to be a moderate predictor of clinical symptoms [12]. However, that study showed significant symptoms among patients without radiographic stenosis, making accurate conclusions from the study challenging.
Deciding to continue conservative therapy or pursue surgery is important for patients with persistent radiculopathy. Our data suggest that patients with a greater degree of stenosis are significantly more likely to experience worse baseline physical functioning. Moreover, preoperative discussions with patients should underscore the likelihood of physical disability in guiding shared decision-making regarding surgical intervention. Importantly, our data also suggest that ACDF will improve patient symptoms regardless of the degree of stenosis but may provide greater self-reported physical function in patients with severe neural foraminal stenosis. Therefore, surgical decisions should be based on the patientŌĆÖs clinical symptoms, including pain, failed conservative management, or neurologic deficits. Conversely, radiographic findings may not be clinically beneficial in recommending against surgical intervention because even patients with minor radiographic stenosis who were indicated for surgery show considerable and comparable surgical improvement.
This study has limitations, particularly those inherent in retrospective studies. One limitation of the study is the lower PROM response rate among our patients. A larger patient cohort could confirm our findings that there is a minimal relationship between neuroforaminal stenosis and motor grade improvement following ACDF. Another limitation is that different surgeons perform physical examinations and may grade motor strength differently. Furthermore, fellows or residents may have performed these examinations because our institution is a teaching hospital. Given this limitation, we evaluate PROMs that the assessing surgeon does not confound. While all patients were seen by and operated on by a spine surgeon at the same institution, patients may have obtained MRI scans at outside institutions that use different MRI machines and have different protocols for generating slice thickness. We do not routinely perform postoperative MRI to reduce healthcare waste and expense. Thus, our study is unable to assess the decompression of neuroforaminal stenosis postoperatively. Our utilization of the maximal foraminal stenosis severity for patients with the multilevel disease is also subject to limitations. The interpretation of PROMs cannot be performed on a per-level basis because it would heavily weight patients with multilevel disease in analysis. As PROMs represent a patientŌĆÖs experience with their disease, the individual levelŌĆÖs effects cannot be assigned a PROM alone. While assigning a patient to a group based on the severity of the worst cervical level may be intuitive, this may not explain all disease presentations. Additionally, variability in baseline PROMs between groups may affect the magnitude of PROM improvement. Moreover, baseline differences between groups may have affected the finding of greater improvement in PCS-12. Further studies are needed to evaluate whether this may have been of greater importance than the degree of neural foraminal compression.
Patients with severe cervical foraminal stenosis may present with worse preoperative physical health. ACDF improves self-reported clinical outcomes in all patients, regardless of neuroforaminal stenosis severity, albeit patients with more severe neuroforaminal stenosis may report greater improvement in physical function than those with moderate neuroforaminal stenosis. Despite this improved physical function, similar improvements in motor function are expected regardless of the preoperative degree of neuroforaminal stenosis.
Notes
Author Contributions
Conception: MJL, TZI, YL, KST, GDS; methodology: TZI, YL, MJL, KST, JH; data collection: JH, CP, TBF, SO, EK, JJM; analysis: TZI, JBF, JH, KST; writingŌĆōoriginal draft: TZI, TBF, CP, JH, MJL, YL; writingŌĆōreview and editing: JAC, IDK, JAR, ASH, ARV, CKK, GDS; project administration: JAC, GDS, CKK; study supervision: JAR, JAC, ASH, CKK, ARV, GDS; and approval of final manuscript: MJL, TZI, YL, KST, JH, CP, TBF, SO, EK, JJM, JAC, IDK, JAR, ASH, ARV, CKK, GDS.
Fig.┬Ā1
Schematic of Kim et al. grading system for cervical neural foraminal stenosis (FS) in cervical axial magnetic resonance imaging. (A, B) Grade 0: no FS with intact nerve width: (A) shows no narrowing of neural foramen, and (B) shows mild narrowing. (C) Grade 1: moderate FS whereby the narrowest width of neural foramen is 51%ŌĆō100% of the extraforaminal nerve root width. (D) Grade 2: severe FS, whereby narrowest width of neural foramen is less than 50% of the extraforaminal nerve root width. Reproduced from Kim S, et al. Korean J Radiol 2015;16:1294-302 [9].
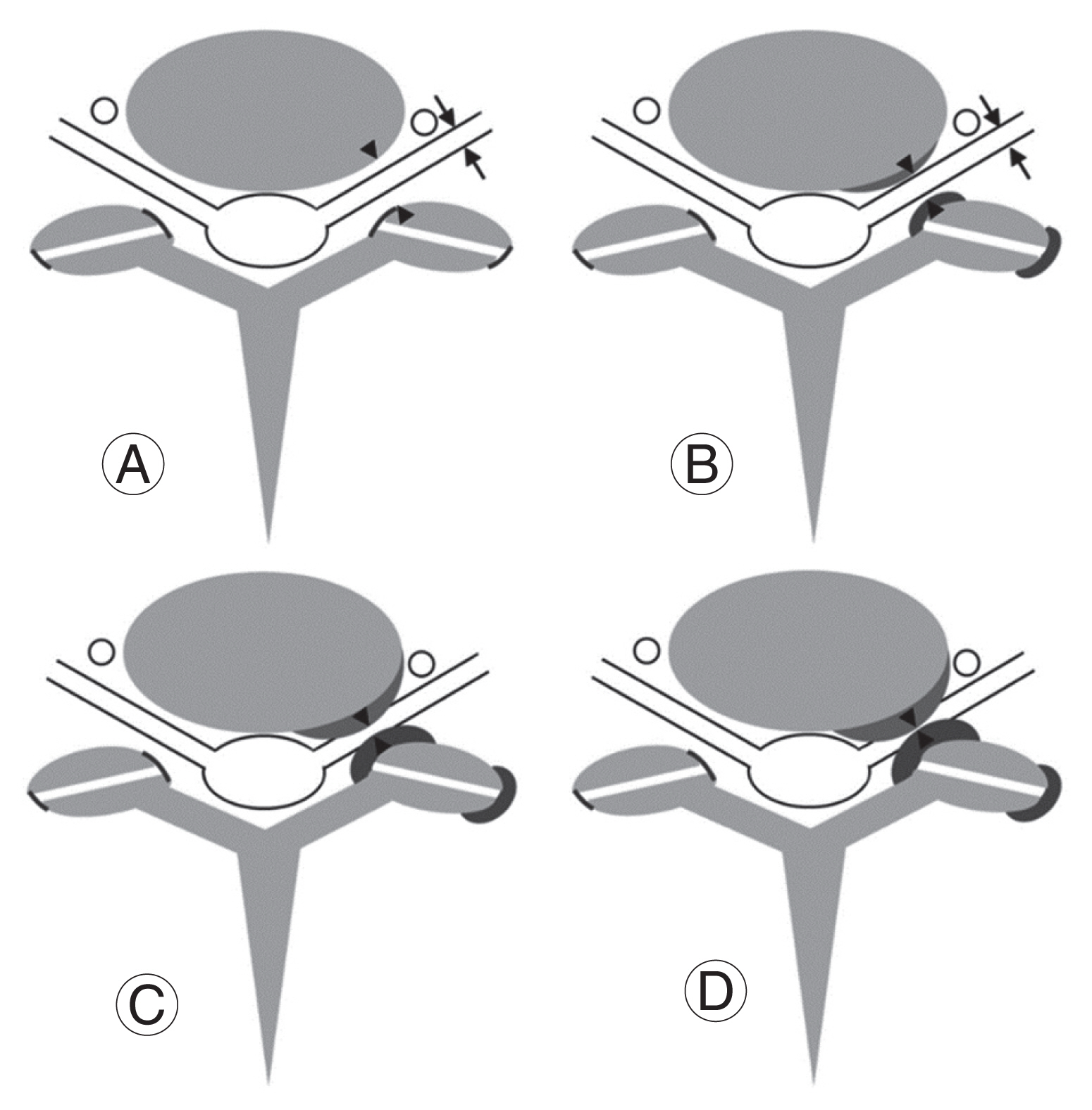
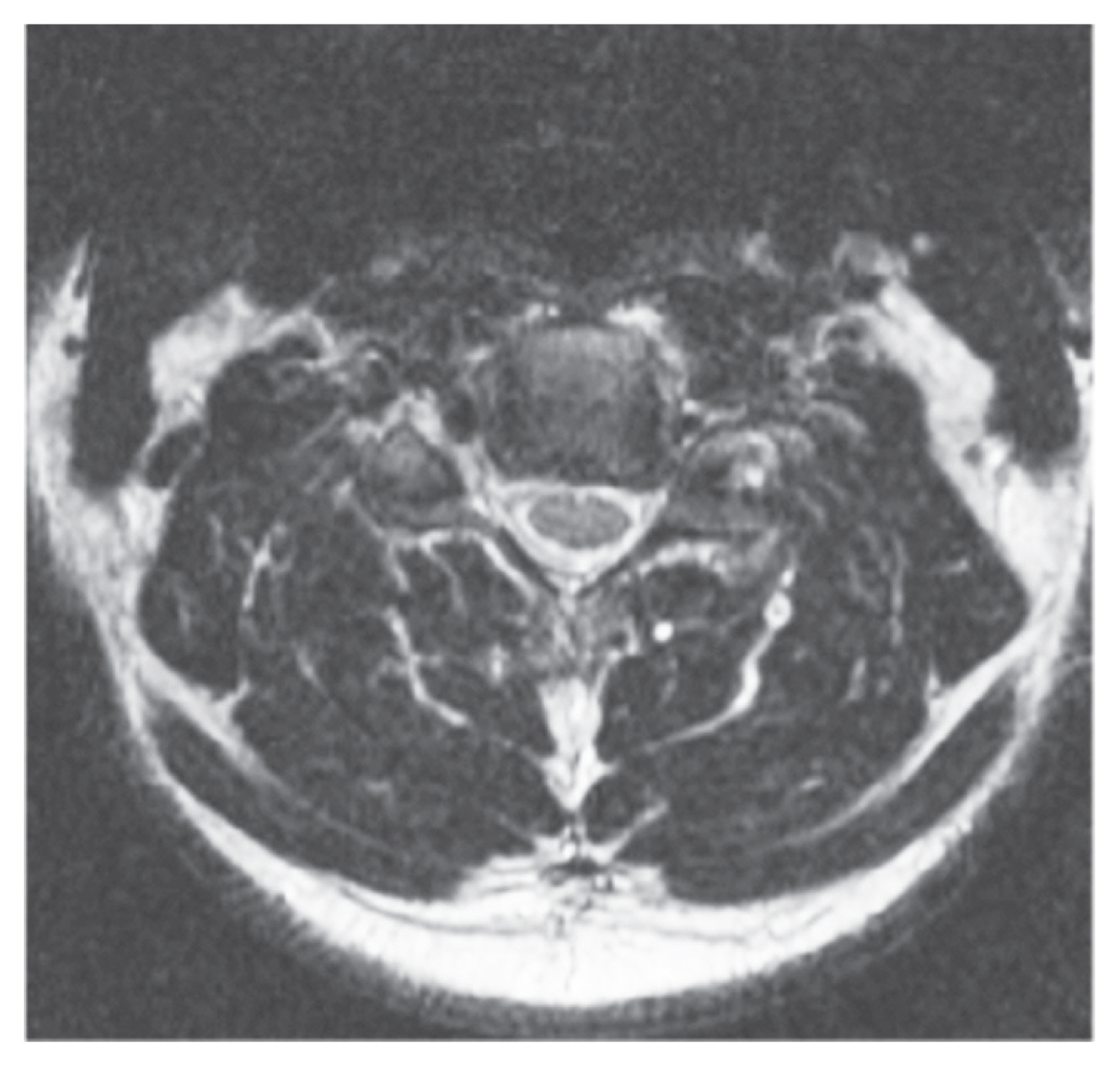
Fig.┬Ā2
A 57-year-old male with patent neural foramina suggesting grade 0 cervical neural foraminal stenosis. Width of the neural foramen equivalent to the extra-foraminal nerve width.

Fig.┬Ā3
A 73-year-old female with grade 1 cervical neural foraminal stenosis. The left neural foramen demonstrates narrowing but is still at least 50% of the width of the extraforaminal nerve root.

Fig.┬Ā4
A 41-year-old male with grade 2 cervical neural foraminal stenosis. In this patient, the narrowest width of the right sided neural foramen is significantly compressed. The width of neural foramen is significantly less than 50% of the width of extraforaminal nerve root.
Table┬Ā1
Demographics based on preoperative stenosis
| Characteristic | Moderate stenosis (N=157) | Severe stenosis (N=197) | p-value |
|---|---|---|---|
| Age (yr) | 52.1┬▒11.8 | 52.0┬▒10.6 | 0.873 |
| Sex | 0.877 | ||
| ŌĆāFemale | 81 (51.6) | 100 (50.8) | |
| ŌĆāMale | 76 (48.4) | 97 (49.2) | |
| Body mass index (kg/m2) | 30.0┬▒5.8 | 30.3┬▒6.5 | 0.705 |
| Elixhauser Comorbidity Index | 1.19┬▒1.4 | 1.44┬▒1.4 | 0.155 |
| Smoking | 0.378 | ||
| ŌĆāNon-smoker | 76 (58.0) | 80 (50.0) | |
| ŌĆāCurrent smoker | 24 (18.3) | 37 (23.1) | |
| ŌĆāFormer smoker | 31 (23.7) | 43 (26.9) | |
| Disease | 0.334 | ||
| ŌĆāRadiculopathy alone | 127 (80.9) | 151 (76.6) | |
| ŌĆāMyeloradiculopathy | 30 (19.1) | 46 (23.4) | |
| Symptom duration | 0.695 | ||
| ŌĆāLess than 6 mo | 69 (43.9) | 79 (40.1) | |
| ŌĆā6 moŌĆō2 yr | 46 (29.3) | 58 (29.4) | |
| ŌĆāMore than 2 yr | 42 (26.8) | 60 (30.5) | |
| Levels fused | <0.001* | ||
| ŌĆāOne level | 87 (55.4) | 60 (30.5) | |
| ŌĆāTwo levels | 59 (37.6) | 119 (60.4) | |
| ŌĆāThree levels | 11 (7.0) | 18 (9.1) | |
| Hospital length of stay (day) | 1.92┬▒3.9 | 1.24┬▒0.4 | 0.107 |
| Discharge | 0.560 | ||
| ŌĆāHome | 88 (97.8) | 102 (99.0) | |
| ŌĆāSkilled nursing facility | 1 (1.1) | 1 (1.0) | |
| ŌĆāInpatient rehab facility | 1 (1.1) | 0 |
Table┬Ā2
Motor function based on preoperative stenosis
| Variable | Weakness Ōēż3/5 | ||
|---|---|---|---|
| Moderate stenosis (N=157) | Severe stenosis (N=197) | p-value | |
| Preoperative motor weakness | 23 (13.5) | 35 (16.5) | 0.431 |
| Postoperative motor weakness | 5 (2.9) | 10 (4.7) | 0.380 |
| At least partial recoverya) | 18 (78.3) | 25 (71.4) | 0.561 |
| Full recoverya) | 20 (87.0) | 34 (97.1) | 0.134 |
Table┬Ā3
Recovery of motor function on an individual level basis based on preoperative stenosis
| Variable | Weakness Ōēż3/5 | ||
|---|---|---|---|
| Moderate stenosis (N=329) | Severe stenosis (N=256) | p-value | |
| Preoperative motor weakness | 45 (13.7) | 33 (12.9) | 0.781 |
| Postoperative motor weakness | 12 (3.6) | 8 (3.1) | 0.730 |
| At least partial recoverya) | 38 (84.4) | 27 (81.9) | 0.758 |
| Full recoverya) | 33 (73.3) | 25 (75.8) | 0.809 |
Table┬Ā4
Patient-reported outcome measures based on preoperative stenosisa)
| Variable | Moderate stenosis | Severe stenosis | p-value |
|---|---|---|---|
| MCS12 Preop | 49.0┬▒10.1 | 44.8┬▒12.5 | 0.105 |
| MCS12 1 year | 50.6┬▒10.4 | 48.0┬▒11.8 | 0.349 |
| MCS12 Δ | 1.58±9.9 | 3.19±12.3 | 0.483 |
| PCS12 Preop | 37.3┬▒9.0 | 33.3┬▒7.8 | 0.049* |
| PCS12 1 year | 38.2┬▒10.4 | 38.6┬▒10.6 | 0.761 |
| PCS12 Δ | 0.87±11.3 | 5.43±10.9 | 0.048* |
| VAS neck Preop | 5.54┬▒2.9 | 6.39┬▒2.6 | 0.235 |
| VAS neck 1 year | 2.29┬▒3.0 | 3.47┬▒2.9 | 0.021* |
| VAS neck ╬ö | ŌłÆ3.26┬▒2.8 | ŌłÆ2.92┬▒3.3 | 0.640 |
| VAS arm Preop | 5.34┬▒3.1 | 6.03┬▒2.3 | 0.558 |
| VAS arm 1 year | 2.57┬▒3.0 | 3.11┬▒2.6 | 0.165 |
| VAS arm ╬ö | ŌłÆ2.77┬▒3.4 | ŌłÆ2.92┬▒3.3 | 0.855 |
Table┬Ā5
Multivariate linear regression analysis for outcome measures
| Predictors | ΔVAS neck | ΔVAS arm | Δ MCS-12 | Δ PCS-12 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| Stenosis grade | 0.69 (ŌłÆ0.88 to 2.28) | 0.378 | 0.42 (ŌłÆ1.30 to 2.15) | 0.625 | 0.57 (ŌłÆ4.11 to 5.24) | 0.810 | 5.59 (0.84 to 10.3) | 0.022* |
|
|
||||||||
| Myeloradiculopathy (Ref: radiculopathy alone) | 1.10 (ŌłÆ0.65 to 2.84) | 0.213 | ŌłÆ0.05 (ŌłÆ1.95 to 1.84) | 0.954 | ŌłÆ2.52 (ŌłÆ7.73 to 2.68) | 0.338 | 2.84 (ŌłÆ2.42 to 8.10) | 0.287 |
|
|
||||||||
| Age | 0.03 (ŌłÆ0.04 to 0.10) | 0.351 | 0.05 (ŌłÆ0.02 to 0.13) | 0.181 | ŌłÆ0.18 (ŌłÆ0.38 to 0.03) | 0.097 | 0.07 (ŌłÆ0.14 to 0.28) | 0.505 |
|
|
||||||||
| BMI | ŌłÆ0.02 (ŌłÆ0.14 to 0.10) | 0.714 | ŌłÆ0.08 (ŌłÆ0.21 to 0.05) | 0.238 | ŌłÆ0.26 (ŌłÆ0.60 to 0.09) | 0.142 | ŌłÆ0.22 (ŌłÆ0.57 to 0.14) | 0.227 |
|
|
||||||||
| Levels | ŌłÆ0.05 (ŌłÆ1.28 to 1.18) | 0.936 | 0.56 (ŌłÆ0.77 to 1.90) | 0.403 | 3.31 (ŌłÆ0.66 to 7.28) | 0.101 | 0.22 (ŌłÆ3.80 to 4.23) | 0.915 |
References
1. Woods BI, Hilibrand AS. Cervical radiculopathy: epidemiology, etiology, diagnosis, and treatment. J Spinal Disord Tech 2015;28:E251ŌĆō9.

2. Broekema AE, Groen RJ, Simoes de Souza NF, et al. Surgical interventions for cervical radiculopathy without myelopathy: a systematic review and meta-analysis. J Bone Joint Surg Am 2020;102:2182ŌĆō96.

3. Kang Y, Lee JW, Koh YH, et al. New MRI grading system for the cervical canal stenosis. AJR Am J Roentgenol 2011;197:W134ŌĆō40.


4. Mansfield M, Smith T, Spahr N, Thacker M. Cervical spine radiculopathy epidemiology: a systematic review. Musculoskeletal Care 2020;18:555ŌĆō67.



5. Epstein NE. A review of complication rates for anterior cervical diskectomy and fusion (ACDF). Surg Neurol Int 2019;10:100.



6. Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310ŌĆō7.


7. Carr FA, Healy KM, Villavicencio AT, et al. Effect on clinical outcomes of patient pain expectancies and preoperative Mental Component Summary scores from the 36-Item Short Form Health Survey following anterior cervical discectomy and fusion. J Neurosurg Spine 2011;15:486ŌĆō90.


8. Massel DH, Mayo BC, Bohl DD, et al. Improvements in neck and arm pain following an anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2017;42:E825ŌĆō32.


9. Kim S, Lee JW, Chai JW, et al. A new MRI grading system for cervical foraminal stenosis based on axial T2-weighted images. Korean J Radiol 2015;16:1294ŌĆō302.



10. Meacock J, Schramm M, Selvanathan S, et al. Systematic review of radiological cervical foraminal grading systems. Neuroradiology 2021;63:305ŌĆō16.



11. Kreitz T, Huang R, Beck D, Park AG, Hilibrand A. Prolonged preoperative weakness affects recovery of motor function after anterior cervical diskectomy and fusion. J Am Acad Orthop Surg 2018;26:67ŌĆō73.


12. Park HJ, Kim JH, Lee JW, et al. Clinical correlation of a new and practical magnetic resonance grading system for cervical foraminal stenosis assessment. Acta Radiol 2015;56:727ŌĆō32.



13. Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol 2015;36:811ŌĆō6.



14. Pinter ZW, Sebastian AS, Wagner SC, et al. Indicators for substantial neurological recovery following elective anterior cervical discectomy and fusion. Clin Spine Surg 2022;35:E698ŌĆō701.


15. Magnus W, Viswanath O, Viswanathan VK, Mesfin FB. Cervical radiculopathy [Internet] Treasure Island (FL): StatPearls Publishing. 2022 [cited 2022 Sep 18]. Available from: https://pubmed.ncbi.nlm.nih.gov/28722858/
16. Saifi C, Fein AW, Cazzulino A, et al. Trends in resource utilization and rate of cervical disc arthroplasty and anterior cervical discectomy and fusion throughout the United States from 2006 to 2013. Spine J 2018;18:1022ŌĆō9.


17. Kim MS, Lee DG, Chang MC. Outcome of transforaminal epidural steroid injection according to severity of cervical foraminal stenosis. World Neurosurg 2018;110:e398ŌĆō403.


18. Shenoy K, Patel PD, Henstenburg JM, et al. Impact of preoperative weakness and duration of symptoms on health-related quality-of-life outcomes following anterior cervical discectomy and fusion. Spine J 2020;20:1744ŌĆō51.


19. Yoo JS, Ahn J, Mayo BC, et al. Improvements in grip and pinch strength and patient-reported outcomes after anterior cervical discectomy and fusion. Clin Spine Surg 2019;32:403ŌĆō8.









